Introduction
Recurrent miscarriage (RM) refers to two or more consecutive pregnancy losses documented by ultrasound or histopathologic examination according to the American Society for Reproductive Medicine (ASRM),1 and affects approximately 2-4% of couples who are trying to conceive.2,3 RM can be linked to parental chromosomal abnormalities, untreated hypothyroidism, uncontrolled diabetes mellitus, certain uterine anatomic abnormalities, antiphospholipid antibody syndrome (APS), additional endocrine disorders, heritable and/or acquired thrombophilia, immunologic abnormalities, infections, embryo or uterine factors, and environmental factors.3,4 Therefore, preimplantation genetic testing for aneuploidy (PGT-A) was developed to select chromosomally normal embryos.5–7 However, these causes account for only half of all cases, with another half of cases remaining unexplained.8
Despite the improvement of the standards of care in IVF to increase the success rate during IVF treatment such as the transfer of euploid embryos, the reported pregnancy rates from euploid embryo transfers are as high as 70% at the most,9 with a possibility for a miscarriage after implantation (30%).10,11 One explanation of the Implant failure is due to problems with endometrial receptivity.12 Research indicates that recurrent implantation failure (RIF) in cases of seemingly normal endometria may be linked to functional and molecular alterations in the uterus, such as aberrant endometrial microbiota (including the existence of chronic endometritis), inadequate synchronization between the blastocyst and endometrium, and/or excessive uterine peristalsis.13 Other elements influencing embryo implantation have been suggested such as immunologic or inflammatory mediators of implantation failure14,15. Other than that, structural problems and Uterine pathologies like myoma, polyp, adenomyosis, and adhesion can cause implantation failure; however clinicians vigorously assess such patients for these pathologic condition with RIF and many of them have history of laparoscopic or hysteroscopic correction and evaluation to exclude such disorders.16,17
Although immunological variables are thought to be crucial for embryo implantation, there is a lot of conflicting data regarding the benefits of immunological treatment for RM and RIF patients.18,19 A hematopoietic-specific cytokine known as Granulocyte Colony Stimulating Factor (G-CSF) is a glycoprotein first identified in mice and later on humans (hG-CSF) was cloned.20,21 G-CSF is an ubiquitous growth factor, found in a wide variety of tissue types, including reproduction organs.22 It modulates the release of neutrophils into the bloodstream promotes their growth and differentiation in the bone marrow, and increases phagocytosis and the oxidative process in mature neutrophils that speeds up the oxidative process and phagocytosis both of which are essential for implantation. G-CSF influences the expression of genes essential for the implantation process, including those involved in cellular adhesion mechanisms, endometrial vascular remodeling, and local immune regulation.23,24 Therefore, it was suggested as a therapeutic agent to enhance endometrial receptivity due to its role in promoting tissue repair, angiogenesis, and immunomodulation.25 Binding to their receptors G-CSF receptors (G-CSFR), present in a few nonhematopoietic cell types, such as endothelium, placenta, trophoblastic, and granolous luteina cells leads to an increase of the endometrial thickness and herein increases in success rates of implantation.19,26–28
By controlling the expression of genes linked to embryo adhesion, cell migration, tissue remodelling, and angiogenesis—all of which are critical for endometrial growth, successful embryo implantation, and the subsequent development of the placenta—G-CSF also plays a crucial role in embryo implantation.29 Furthermore, because pregnancy poses an immunological challenge to the mother because of the fetus’s semi-allogenic origin, G-CSF may play a role in promoting adaptive alterations that support immune tolerance throughout pregnancy. Important components of the immunoregulatory activities throughout the implantation period, G-CSF promotes the development of tolerogenic dendritic cells and regulatory T-cells that produce IL-10 [50] and shifts the T-cell cytokine secretion profile to Th2 responses.30
G-CSF is increasingly explored for its potential role in assisted reproduction and pregnancy. While the exact mechanism by which G-CSF may improve IVF outcomes is not fully understood, it is believed to have anti-inflammatory and immunomodulatory effects that can promote a receptive uterine environment for embryo implantation in mice and humans23,31 In the reported cases here, we used intrauterine G-CSF for several patients who had experienced multiple RIF or RM after their assisted conception treatment and noticed an improvement in the pregnancy outcome. Thus, we evaluate the clinical effect of G-CSF on the outcome of these patients with RIF & RM such as ongoing pregnancy, abortion, and implantation rates. While it has been extensively studied in neonates and for conditions like chronic neutropenia during pregnancy, its safety and efficacy in the context of assisted reproductive technologies (ART) remain underexplored. This study seeks to address the gap in evidence regarding the impact of G-CSF administered prior to embryo transfer, particularly its potential implications for fetal development and pregnancy outcomes.
Materials and Methods
Patient selection
This study was conducted at Fakih IVF Center, Abu Dhabi, UAE, between April 2023 and January 2024. A total of 19 patients were included, selected based on consultations during this period.
Patient characteristics
The Age Ranged between 27–44 years and Body Mass Index (BMI): 24.6–38.5 kg/m².
Treatment protocol
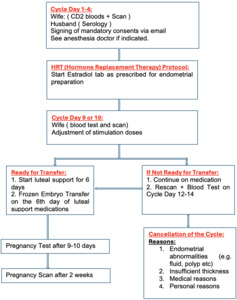
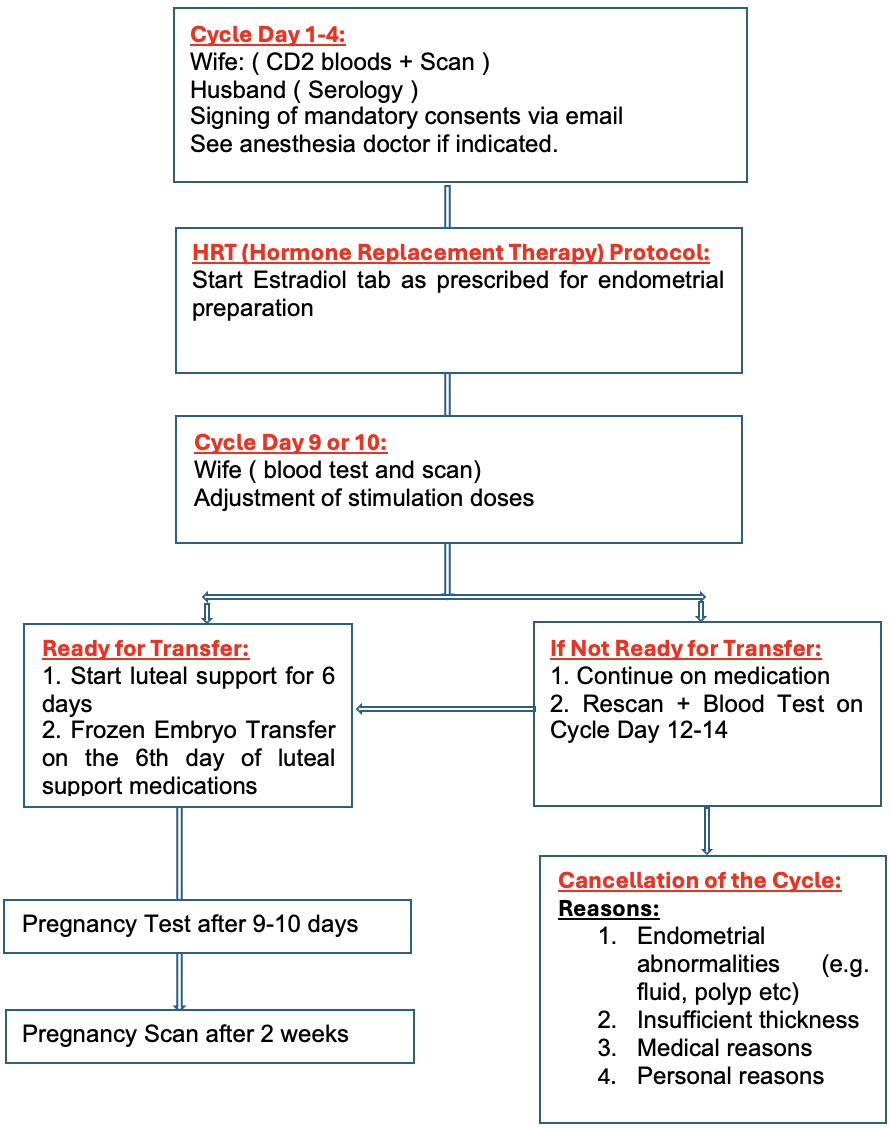
The frozen embryo replacement cycle included the HRT stimulated cycle. After undertaking a urine human chorionic gonadotropin (hCG) test, a transvaginal scan was performed on the second day of menstruation. Baseline hormones, serum FSH, LH Estradiol, and progesterone were undertaken. Then, oral estradiol valerate tablets (4–6 mg/d, Bayer, Leverkusen Germany) were started. Vaginal ultrasound was performed 10 days later to measure the endometrium thickness. If the endometrium thickness reached 7 mm or more as assessed by transvaginal scan then intrauterine infusion of Filgastrim (G-CSF) 300mcg/5ml was administered using an embryo transfer catheter. The transfer was canceled if the serum progesterone value was more than 1.5 ng/ml. After starting the luteal support, a Single Euploid blastocyst was scheduled on D6 (if the day commencing progesterone was D0) under transabdominal ultrasound guidance as described before.32 All patients subsequently received our standard luteal support that includes, oral estradiol tablets in escalated doses, transvaginal progesterone, Intramuscular progesterone in oil every three days, oral Duphaston and Clexane 40 mg subcutaneously once a day. In addition to continuing the intramuscular injection of progesterone (60 mg daily) and estrogen, 20 mg of dydrogesterone was added daily until 10 weeks of gestation. Blood hCG was measured 12 days after transfer. If positive, then they would have an ultrasound to assess clinical pregnancy. The dose of estrogen was stopped in case viable pregnancy was confirmed by ultrasound and the dose of progesterone was continued till 12 weeks of gestational week.
Outcome measures
Pregnancy outcomes were assessed based on positive serum βhCG test (biochemical pregnancy), positive fetal heart by transvaginal ultrasound examination 10 days post-embryo transfer (clinical pregnancy), miscarriage, and ongoing viable pregnancy.
Data Collection and Analysis
Data were collected retrospectively from patients’ electronic records. The outcomes were analyzed to determine the efficacy of G-CSF infusion in improving pregnancy rates and reducing miscarriage rates among the study participants.
Results
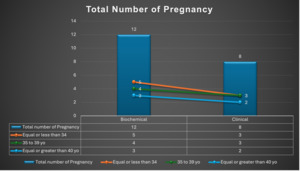
In this study, 19 patients received intrauterine G-CSF infusion as part of their IVF treatment. Of these, 12 patients (70.5%) tested positive for pregnancy based on serum βhCG levels. Among the pregnant patients, 8 (66.7%) achieved ongoing pregnancies, while 2 (16.6%) experienced first-trimester miscarriages, and 2 (16.6%) had biochemical pregnancies.
On the other hand, 5 patients (29%) had negative pregnancy tests, and 2 patients had their embryo transfer canceled due to bleeding. Notably, none of the patients developed any side effects related to the G-CSF infusion.
Discussion
According to the results of our case series, patients undergoing IVF who have a history of repeated implantation failure or loss may benefit from intrauterine Granulocyte-Colony Stimulating Factor (G-CSF) infusion. For patients with repeated implantation failure (RIF) or recurrent miscarriage (RM), G-CSF has shown encouraging promise as an adjuvant therapy in assisted reproductive technologies (ART). Through processes that aid in embryo implantation and pregnancy maintenance, including immune modulation, cytokine control, and angiogenesis, G-CSF increases endometrial receptivity.33
Implantation failures and RM are linked to immunological variables, including CD4+CD25+ T-cell dysfunction, natural killer (NK) cell cytotoxicity, HLA incompatibility, and an imbalance between T-helper cell subsets (32). To address these problems, a number of immunomodulatory therapies have been investigated, such as lipid infusions, intravenous immunoglobulins, corticosteroids, lymphocyte immunotherapy, seminal plasma, and anti-TNF drugs.34–39 However, G-CSF has become a more focused strategy.
Numerous research back up G-CSF’s ability to enhance pregnancy outcomes. For example, Santjohanser et al. found that patients receiving G-CSF had greater rates of pregnancy (47%) and live birth (32%) than patients in a placebo group (27% and 14%, respectively; p=0.016 and p=0.006).40 Gleicher et al. discovered that intrauterine G-CSF increased endometrial thickness in individuals with thin endometrium, and all but one patient experienced successful implantation results.41 Furthermore, in a randomised, double-blind, placebo-controlled study of 100 RIF patients, the G-CSF group showed improved endometrial thickness in both fresh and frozen cycles and significantly higher chemical pregnancy rates than the placebo group.42 These results highlight G-CSF’s potential as an ART adjunct therapy, especially for individuals with for patients with challenging reproductive histories.
The mechanism by which G-CSF improves endometrial receptivity appears to involve gene expression modulation, enhancing cellular adhesion, vascular remodeling, and immune regulation.43 Furthermore, G-CSF increases regulatory T cells and dendritic cells, promoting a balanced immune environment conducive to implantation.44 Animal models also highlight its anti-abortive effects.45 However, not all studies report consistent outcomes, which may be attributed to variations in dosing regimens and study designs. For example, lower doses (100 µg) used in some cohorts were associated with no substantial improvement in pregnancy outcomes compared to higher doses.46 Additionally, non-randomized study designs and small sample sizes limit the generalizability of findings.47
G-CSF appears to increase cellular adhesion, vascular remodeling, and immunological control through modulating gene expression, which in turn increases endometrial receptivity.43 Additionally, G-CSF promotes a balanced immunological milieu that is favorable for implantation by increasing dendritic cells and regulatory T cells.44 Its anti-abortive properties are also demonstrated in animal models.45 However, because to differences in study designs and dosage schedules, not all studies show consistent results. For instance, compared to greater dosages, lesser doses (100 µg) administered in some cohorts did not significantly enhance pregnancy outcomes.46 Furthermore, limited sample numbers and non-randomized study designs restrict how broadly the results may be applied.47
Patients treated with intrauterine G-CSF in conjunction with progesterone and anticoagulant medication (Clexane 40 mg) showed positive results in our study. Results for patients who had previously had unsuccessful cycles improved, indicating that G-CSF played a role in their success. However, the tiny sample size and absence of randomization make it impossible to draw firm conclusions. More extensive randomized controlled studies are required to confirm these results and develop the best practices. Crucially, G-CSF had no negative effects in either our study or any other, suggesting a good safety profile. By stimulating the immunological pathways involved in implantation or causing mild inflammation to increase endometrial receptivity, G-CSF may improve implantation.48 Additionally, it promotes adhesion, apposition, and invasion by facilitating contacts between the embryo and endometrium.49
Despite these promising findings, concerns remain regarding the safety of G-CSF during pregnancy due to its ability to cross the placenta. Potential risks include spontaneous miscarriage and congenital malformations,50 which are thought to stem from differences in fetal metabolism and immature organ systems.51 Nevertheless, four large studies and five case reports encompassing 162 pregnancies showed no increased incidence of fetal death or congenital malformations when G-CSF was used for chronic neutropenia during pregnancy.52–55 In our study, G-CSF was administered six days before blastocyst transfer during IVF. Given its short half-life (3.5–3.8 hours) and renal clearance, the drug is likely eliminated from the system before implantation occurs, minimizing potential fetal exposure.56
Current evidence supports the safety and efficacy of G-CSF in improving IVF outcomes, though long-term safety data remain limited. Further research is required to assess neonatal health, developmental milestones, and overall pregnancy outcomes. Large-scale, randomized controlled trials are essential to confirm these findings and explore the precise mechanisms by which G-CSF enhances endometrial receptivity and implantation success.
In conclusion, intrauterine G-CSF infusion holds promise as a therapy for improving pregnancy outcomes in patients with RIF or RM undergoing IVF. Its administration before implantation appears safe due to rapid drug clearance, but ongoing research is crucial to establish its long-term safety and efficacy. While G-CSF appears to improve ART outcomes without significant risks, caution remains warranted, and standardized protocols need to be developed for its integration into clinical practice.
Conclusion
Intrauterine G-CSF infusion shows clinical promising results in enhancing pregnancy outcomes for patients with a history of recurrent miscarriage or recurrent implantation failure undergoing IVF treatment. Further large-scale RCTs & more research are warranted to confirm these findings and develop standardized protocols for its integration within novel therapeutic strategies to improve IVF success rates. Moreover, whilst current evidence suggests that G-CSF use during pregnancy, especially in the context of IVF, is not associated with significant risks, caution remains warranted. Its administration before implantation appears safe due to rapid drug clearance, but ongoing research is essential to validate its safety and efficacy in the long term.
Funding Statement
No external funding for this study
Competing Interest
None
Authors’ contribution – per CRediT
Conceptualization: Dr. Yasmin Sajjad
Data curation: Dr. Marwa Alhamoudi
Formal Analysis: Rona Mae Nad
Investigation: Dr. Marwa Alhamoudi
Methodology: Dr. Yasmin Sajjad
Software: Rona Mae Nad
Supervision: Dr. Fakih
Validation: Dr. Yasmin
Writing – original draft; Dr. Marwa Alhamoudi, Dr. Nermeen Soliman.
Writing – review & editing: Dr. Nermeen Soliman, Dr. Yasmin Sajjad
Submission declaration
-
The manuscript has never been published before.
-
There are no other publications considering it.
-
The publication is approved, either expressly or implicitly, by all authors and the relevant authorities where the work was completed.