Introduction
Frozen embryo transfer (FET) has become a cornerstone of modern assisted reproductive technology (ART). Over the last twenty years, the frequency of FET has risen markedly compared with fresh transfers, largely due to the improved technique and routine use of vitrification, enhanced post-thaw embryo survival, and recognition of the clinical advantages associated with deferred transfers. These advantages include minimizing the risk of ovarian hyperstimulation syndrome (OHSS), providing greater flexibility in scheduling embryo transfer, and potentially improving obstetric and neonatal outcomes.
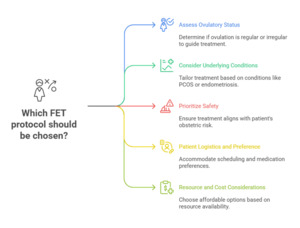
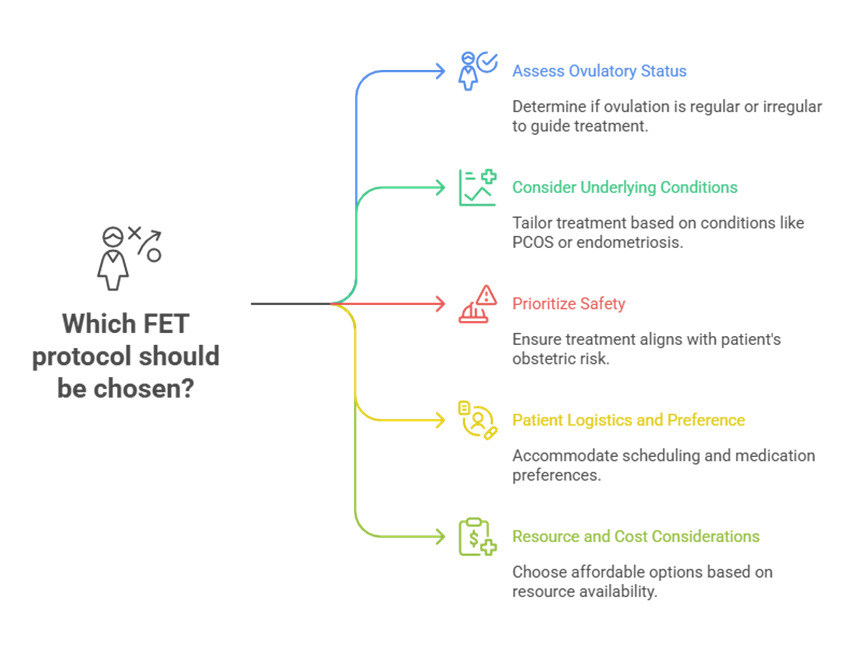
The success of FET is critically dependent on precise synchronization between embryonic developmental stage and endometrial receptivity, a period known as the “window of implantation” (WOI). Consequently, the strategy adopted for endometrial preparation plays a decisive role in determining implantation and live birth outcomes. Increasingly, it is recognized that no single approach suits all patients, and that individualization of endometrial preparation based on patient characteristics, hormonal milieu, comorbidities and prior treatment response represents the real key to optimizing FET success. Patient-centered considerations such as convenience, treatment burden, safety profile, and personal preference also play a pivotal role in determining the most appropriate protocol. (Fig. 1)
Traditionally, endometrial priming for FET has relied on two principal modalities: hormone replacement therapy (HRT) or artificial cycles, and natural cycles (NC), either spontaneous or modified with ovulation induction or trigger. Both have well-established roles, but limitations remain, which has led a necessity for the development of alternative variations and new protocols. Recent emphasis has shifted towards regimens that not only enhance implantation and pregnancy rates but also reduce treatment-related burden, improve maternal safety, and align more closely with physiological mechanisms. The search for improved protocols is underpinned by several factors. First, despite advances in embryo cryopreservation and survival, implantation failure persists , particularly in patients with recurrent implantation failure (RIF), thin endometrium, or coexisting conditions such as polycystic ovary syndrome (PCOS) and endometriosis. Second, published evidences indicate that the absence of a functional corpus luteum in HRT cycles may predispose patients to complications, including hypertensive disorders of pregnancy, abnormal placentation, and related obstetric risks. Third, patient-centered approaches increasingly prioritize methods that simplify treatment, minimize adverse effects, and promote patient comfort without compromising efficacy.
Considering all these factors, several innovative approaches to endometrial preparation have been introduced. Among them are letrozole-stimulated cycles, which may provide a balance between natural and artificial methods by promoting ovulation while preserving corpus luteum function; downregulation and ovulation induction (DROI) regimens, designed to improve endometrial receptivity through downregulation followed by individualized luteal support; and a variety of adjunctive strategies such as intrauterine platelet-rich plasma (PRP) infusion and immunomodulatory interventions. Drawing upon data from randomized controlled trials (RCTs), meta-analyses, and large observational studies, this Article will explore these emerging protocols, review the evidences supporting their use, and outline clinical contexts in which individualized strategies may offer particular advantages compared with conventional approaches.
Materials and Methods
This review was conducted as a narrative synthesis of the current evidences available on endometrial preparation strategies for Frozen Embryo Transfer (FET) cycles. A comprehensive literature search was performed using the PubMed, Scopus, and Cochrane Library databases to identify relevant studies published up to December 2025. The search strategy incorporated combinations of keywords and MeSH terms including “frozen embryo transfer,” “endometrial preparation,” “natural cycle,” “artificial cycle,” “HRT,” “letrozole-stimulated cycle,” “GnRH agonist,” “DROI,” “implantation,” “pregnancy outcomes,” and “individualized protocols.”
Both randomized controlled trials (RCTs) and observational studies (prospective or retrospective cohorts, case-control studies) were included. Meta-analyses, systematic reviews, and key narrative reviews were also examined to ensure comprehensive coverage of available evidence. Articles were included if they were published in English and reported clinical, hormonal, or reproductive outcomes relevant to FET or endometrial receptivity. Studies focusing on animal models, unrelated gynaecological conditions or lacking outcome data were excluded. Reference lists of selected publications were manually screened to identify additional relevant studies. Preference was given to high-quality evidence, particularly multi-centric RCTs and meta-analyses, while also incorporating recent real-world data to reflect evolving clinical practice. The extracted data were synthesized qualitatively, emphasizing comparative outcomes, physiologic rationale, and patient-centered approach influencing the protocol selection. No ethical approval was required, as this work involved the review and synthesis of previously published literature.
Results
As this is a narrative review, the findings are synthesized from published randomized controlled trials (RCTs), meta-analyses, and large observational studies rather than presented as original data. The results are organized according to the major endometrial preparation approaches used in frozen–thawed embryo transfer (FET) cycles, with comparative emphasis on clinical outcomes, physiologic mechanisms, and patient-centered implications.
1. Hormone Replacement Therapy (HRT) or Artificial Cycle
Most contemporary studies, including multiple RCTs and meta-analyses, demonstrate that HRT cycles achieve comparable implantation and live birth rates to natural cycles (NC), particularly when luteal phase support is optimized. However, the absence of a functional corpus luteum has been consistently linked to higher incidences of abnormal placentation, hypertensive disorders of pregnancy, and altered angiogenic profiles. Despite its convenience and scheduling flexibility, HRT cycles may therefore be less physiologic and may carry a slightly higher risk of obstetric complications in some cohorts but conclusive evidences are still insufficient to prove such complications.
2. Natural Cycle (NC) and Modified Natural Cycle (mNC)
Natural cycles, either spontaneous or modified by hCG trigger, preserve corpus luteum function and maintain physiological hormonal balance. Evidence from large meta-analyses indicates similar live birth rates compared to HRT cycles, with potential advantages in obstetric outcomes and patient satisfaction. However, the main limitation remains the unpredictability of ovulation and the logistical challenge of monitoring, particularly in irregular or anovulatory women.
Li et al. found that natural-cycle FET achieved higher live-birth rates and substantially lower pregnancy loss than HRT, even after adjusting for confounders. These benefits were consistent across all subgroups, indicating a robust advantage of the natural cycle. The authors conclude that, despite the convenience of HRT, the natural cycle may be prioritized for single euploid blastocyst transfers.1
One large prospective randomized non-inferiority trial conducted by Groenewoud et al in 2016, compared artificial-cycle FET with modified natural-cycle FET in 959 women aged 18–40 years. Live birth, clinical pregnancy, and ongoing pregnancy rates were statistically similar between the two protocols, confirming that artificial-cycle preparation is not inferior to a modified natural cycle for FET outcomes. Although reproductive success rates were comparable, artificial cycles showed a significantly higher cancellation rate. Cost analysis demonstrated no meaningful difference in per-cycle expenses between the two approaches. Despite slightly fewer participants than originally planned, the trial supports the use of either method, with clinical decision-making guided by patient convenience and cycle management considerations.2
3. Letrozole-Stimulated Cycles
Letrozole-induced cycles have emerged as an appealing middle ground between NC and HRT. By stimulating mono-follicular growth and supporting corpus luteum development, it combines the advantage of physiological hormonal milieu with scheduling control. Evidences from RCTs and recent meta-analyses demonstrate comparable or slightly improved live birth and clinical pregnancy rates versus HRT cycles, with reduced hypertensive risk and higher patient acceptability. However, long-term obstetric outcomes require further validation in large-scale trials. A multicenter randomized trial including 155 PCOS women found that the letrozole ovulation regimen with programmed FET produced similar clinical pregnancy, live-birth, and miscarriage rates, in the single-blastocyst FET subgroup. Obstetric and neonatal outcomes were also comparable. The only meaningful difference was that letrozole cycles required less intensive luteal support, suggesting a potentially simpler clinical workflow without compromising success.3 however, the evidences are limited and Large multicenteric RCTs are required to prove the safety and efficacy.
4. Depot GnRH Agonist (DROI) Protocols
The DROI approach i.e. downregulation followed by ovulation induction and individualized estradiol and luteal supplementation has shown promising results in limited studies, suggesting improved endometrial receptivity and implantation potential. Small RCTs and cohort studies report higher clinical pregnancy and ongoing pregnancy rates compared to standard HRT, but data remain heterogeneous and lack large confirmatory trials. Thus, current evidence for DROI remains preliminary and warrants further investigation.4,5
5. Adjunctive and Experimental Strategies
Adjunctive therapies such as intrauterine platelet-rich plasma (PRP) infusion, granulocyte colony-stimulating factor (G-CSF), and immunomodulatory interventions have been explored for patients with refractory thin endometrium or repeated implantation failure (RIF). While early studies and small RCTs report encouraging improvements in endometrial thickness and implantation rates, the overall evidence base is weak, and standardized protocols are lacking. Therefore these modalities should be considered experimental , pending for further high-quality data.
Discussions
Endometrial Preparation for Frozen Embryo Transfer:
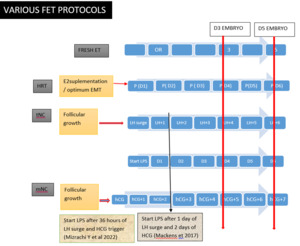
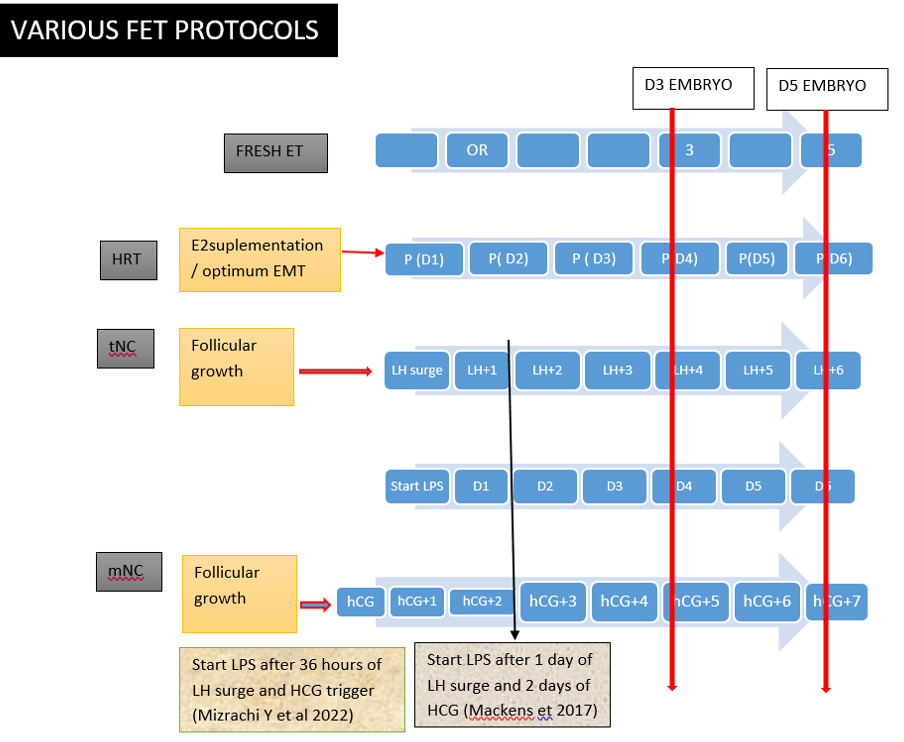
Established Protocols and Recent Modifications - Endometrial preparation for FET is broadly categorized into two major strategies: the artificial or hormone replacement therapy (HRT) cycle and the natural cycle. (Fig. 2)
1. Artificial (HRT) Cycle
This approach relies on the exogenous administration of estradiol to stimulate endometrial proliferation, followed by the introduction of progesterone to replicate the luteal phase and establish the implantation window. Pre-treatment with gonadotropin-releasing hormone agonists (GnRHa) may be used to suppress pituitary activity, though this is not mandatory in all cases.
Estrogen Priming - Adequate estrogen exposure is essential to achieve appropriate endometrial proliferation and expression of progesterone receptors, which are crucial for implantation. Estradiol can be administered using two common regimens:
-
Fixed-dose regimen: A constant daily dose (commonly 6 mg) is given throughout the proliferative phase, aiming to suppress follicular growth and spontaneous ovulation.
-
Step-up regimen: The dose begins at 2 mg and is gradually increased to 6 mg over 10–15 days, mimicking physiological estrogen rise during the natural cycle.
Most guidelines recommend maintaining estrogen supplementation for 10–14 days before adding progesterone in HRT cycles. Prolonged exposure up to 14–21 days has also been associated with a more mature and stable endometrial environment. Shorter durations (<7–10 days) are linked with suboptimal endometrial receptivity, asynchrony with embryo development, and higher miscarriage rates despite seemingly adequate thickness. Wei et al. (2024) reported that abbreviated estrogen exposure (<10 days) can disrupt endometrium–embryo synchrony, contributing to increased early pregnancy losses.6 Both Cochrane reviews and randomized studies have shown that the route of estrogen administration (oral, transdermal, or vaginal) does not significantly affect live birth rates, provided sufficient endometrial proliferation is achieved (≥7–8 mm). No specific preparation (valerate vs. hemihydrate) has demonstrated superiority.
Progesterone Administration - Progesterone replacement is critical in HRT-FET cycles due to the absence of corpus luteum. It can be administered orally, vaginally, intramuscularly (IM), or subcutaneously (SC), each with distinct pharmacokinetic and tolerability profiles. A prospective cohort study by Vuong et al. (2021) evaluated whether adding oral dydrogesterone to vaginal micronized progesterone improves luteal support in FET cycles. Among 1364 women, the combination regimen showed a higher live birth rate (significant after multivariate adjustment) and a significantly lower miscarriage rate compared with vaginal progesterone alone. However, newborn birth weights were lower in the combination group. Despite limitations of its non-randomized, open-label design; the study suggests that adding dydrogesterone may enhance pregnancy outcomes in FET cycles.7
The optimal interval between progesterone initiation and blastocyst transfer remains debated, but most protocols employ 5–7 days of progesterone before transfer. Timing the embryo according to “P + embryonic age” remains the preferred approach to maximize synchrony. Evidence suggests that low serum progesterone (<10 ng/mL) at the time of embryo transfer is associated with reduced pregnancy success. Rescue progesterone supplementation in women with low levels has been shown to restore outcomes comparable to those with adequate luteal support.8,9 Adding SC progesterone to vaginal and oral regimens has yielded promising results, particularly in cycles involving single euploid embryo transfer.
Progesterone threshold controversies
In the programmed FET cycles due to the lack of corpus luteum and hence endogenous progesterone , it becomes a very important aspect to maintain a sufficient level of progesterone by exogenous supplementation of progesterone to tolerate pregnancy and for immunomodulation.
When it comes to use and monitoring of progesterone ( P4) during FET cycles, it is always a matter of debate. Patients and clinicians often weigh pros and cons of natural versus medicated approach. The route of progesterone has always been a key consideration with the options including vaginal , intramuscular and oral routes. There is no universal consensus on optimal thresholds , protocol for monitoring , burden on patients and clinics and potential implications of different levels of progesterone on the day of FET.
Lack of universal threshold
Various studies have suggested different serum P4 levels as the threshold on the day of FET, stating the range from 8 -11 ng/ml. below this level, there are possibilities of higher rate of miscarriages or lower clinical pregnancy or live birth rates. However, there is always a lack of consensus on a single universally agreed-upon cut-off value due to different study designs and study populations, lack of standardisation in the P4 measurement , different timing of measurement and routes of administration.
Low vs high progesterone debate
Most of the studies have concluded that inadequate P4 levels in HRT-FET cycles negatively affect the ART outcome, If P4 levels are very low e.g. lower than 7.8 ng/ml or 5 ng/ml on the day of FET, when compared with the values in optimal range. A retrospective cohort analysis of 236 women undergoing frozen embryo transfer between 2021 and 2023 evaluated the relationship between serum progesterone levels on the day of transfer and reproductive outcomes using fixed thresholds, quartile stratification, and receiver operating characteristic derived cut-offs. While quartile-based comparisons did not show significant differences, progesterone concentrations below 10 ng/mL were associated with significantly lower rates of biochemical pregnancy, implantation, and clinical pregnancy. ROC analysis identified an optimal progesterone threshold of approximately 9.3 ng/mL, above which women demonstrated markedly higher biochemical pregnancy, implantation, clinical pregnancy, ongoing pregnancy, and live birth rates, and this association remained significant after multivariable adjustment. These findings underscore the importance of monitoring luteal progesterone levels and support the use of individualized, center-specific thresholds to optimize outcomes in frozen embryo transfer cycles.10
Regarding high levels of progesterone, also there is a debate , where some studies suggest that more than 32.5ng/ml levels do not lead to improved outcomes. Rather very high levels could be detrimental and may cause asynchrony between embryo and endometrium leading to disturbed cross-talk and molecular dialogue between the two. However some studies found that LBR is not affected negatively even with high P4 levels when a rescue protocol was used to maintain adequate threshold of P4 on the day of FET. Yusuf et al in 2024 , in a large retrospective cohort of artificial FET cycles concluded that serum progesterone levels above 40 ng/mL on the day of embryo transfer did not negatively affect clinical pregnancy or live birth outcomes. Optimal CPR was observed with progesterone levels between 20–40 ng/mL, but higher levels showed no detrimental “ceiling effect.” Embryo quality particularly blastocyst expansion and inner cell mass grade remained the strongest predictors of success. The findings suggested that progesterone dose reduction is unnecessary even when serum levels exceed 40 ng/mL.11
One large retrospective study of over 7500 HRT-FET cycles by Mauro et al in 2024 , found that elevated serum progesterone levels (>40 ng/mL) on the day of embryo transfer do not reduce the likelihood of ongoing pregnancy or live birth. While higher P4 levels showed a slight, non-significant trend toward lower pregnancy and higher miscarriage rates, adjusted analyses confirmed no detrimental effect. Outcomes were comparable across all progesterone groups, including those with levels between 20–40 ng/mL. Overall, high progesterone levels on FET day do not impair live-birth potential in artificial endometrial preparation cycles.12
Monitoring burden vs side-effect
Monitoring of P4 in all FET cycles routinely can create a burden on the clinics and patients. It will lead to multiple blood draws for the patient, cost to patient/clinic, anxiety and fear of reports. Stepping towards individualisation of luteal phase support , designed on the basis of response in previous FET cycles ( if known), initial P4 values , and accordingly deciding the route of administration of progesterone may be a patient friendly approach.
GnRH Agonist + HRT Cycles
Although spontaneous follicular growth and ovulation during HRT cycles are rare (1.9–7.4%), they may lead to cycle cancellation. While randomized trials and meta-analyses show no significant difference in pregnancy or live birth rates with or without GnRHa pretreatment, its use can be valuable in certain clinical settings. For example, in endometriosis, GnRH agonist downregulation may improve receptivity by overcoming progesterone resistance associated with BCL-6 overexpression. Similarly, in Adenomyosis, GnRHa therapy can reduce lesion size and inflammation, thereby enhancing implantation potential. Niu et al in 2013 have reported improved pregnancy outcomes after 2–3 months of GnRHa pretreatment, though results remain inconsistent, particularly in milder disease stages.13
Benefits and Limitations of HRT Cycles
The principal advantage of HRT cycles is schedule flexibility, allowing precise timing of transfer independent of spontaneous ovulation. This makes it particularly suitable for women with irregular or anovulatory cycles, such as those with PCOS, hypothalamic amenorrhea, or diminished ovarian reserve. However, HRT cycles lack the physiological contributions of the corpus luteum, including secretion of vasoactive factors like relaxin, prorenin, and VEGF, which support placental development and maternal adaptation. Large cohort studies and meta-analyses suggest higher rates of hypertensive disorders of pregnancy, preeclampsia, and abnormal placentation in programmed cycles compared with natural or modified natural cycles.14 These limitations have fueled interest in natural and modified natural cycle protocols.
2. Natural Cycle (NC)
In contrast to artificial cycles, the natural approach relies on the woman’s endogenous hormonal pattern to prepare the endometrium.
-
True natural cycle (tNC): Monitoring is performed via urinary LH kits or serial ultrasound until spontaneous ovulation occurs, after which the timing of embryo transfer is adjusted.
-
Modified natural cycle (mNC): Ovulation is triggered pharmacologically with hCG when the leading follicle reaches the desired size, improving predictability of transfer.
Alonso et al. (2024) evaluated outcomes in mNC cycles where hCG was administered at different follicular sizes (13–15.9 mm, 16–18.9 mm, and ≥19 mm). While unadjusted data favored smaller follicles, statistical significance was lost after correcting for confounders. Their findings support the use of hCG trigger within a 13–22 mm follicle size range, provided progesterone levels remain <1.5 ng/mL and endometrial parameters are favorable, offering a practical 6–7 day scheduling window.15
The study by Zhang et al. (2025) retrospectively analyzed 14,431 modified natural-cycle FETs (2013–2023) to determine whether dominant follicle size at hCG trigger (ranging from <12 mm to ≥18 mm) influences reproductive or perinatal outcomes when intensive luteal phase support is used. Across all eight follicle-size categories, live birth, implantation, clinical pregnancy, and pregnancy-loss rates were statistically comparable to the ≥18 mm reference group, even after adjustment for key confounders. No increase in obstetric or perinatal complications was observed in any follicle-size subgroup. These findings indicate that follicle size as small as >10 mm does not compromise outcomes in modified NC-FET cycles when robust luteal support is provided. The authors suggest that this flexibility in trigger timing may simplify monitoring, improve patient convenience and maintain safety without compromising success rates.16
A large single-centre open-label randomized controlled trial from China including 902 ovulatory women undergoing frozen embryo transfer compared natural cycle (NC) with hormone replacement therapy (HRT) endometrial preparation and demonstrated significantly higher live birth rates with NC (54% vs 43%; absolute difference 11.1%, RR 1.26, 95% CI 1.10–1.44), along with lower miscarriage (RR 0.61) and antepartum haemorrhage rates (RR 0.63), while other obstetric and perinatal outcomes were similar between groups. However, interpretation of these findings is limited by the substantial protocol cross-over between treatment arms, as a proportion of women randomized to NC required HRT because of anovulation and vice versa, which may have diluted true between-group differences and reduces certainty in attributing outcomes solely to the assigned endometrial preparation strategy.17 A large retrospective propensity-matched cohort study including 2365 frozen embryo transfer cycles (1892 hormone replacement therapy [HRT] and 473 modified natural cycle [mNC]) in ovulatory women compared these two endometrial preparation strategies under intensive luteal phase support with vaginal progesterone and oral dydrogesterone. After adjustment for baseline characteristics, live birth rates were virtually identical between the mNC and HRT groups (approximately 35% in both arms; adjusted RR close to 1.0 with confidence intervals spanning unity), with no meaningful differences in overall pregnancy, clinical pregnancy, ongoing pregnancy, miscarriage, multiple gestation, gestational age at delivery, or neonatal birthweight. These findings suggest that when adequate luteal support is provided, both HRT and mNC protocols may achieve comparable reproductive and perinatal outcomes in ovulatory women undergoing FET. However despite its large sample size, this study has several important limitations. Its retrospective design introduces the potential for residual confounding despite propensity score matching, and causal inference therefore remains limited compared with randomized controlled trials. Selection bias cannot be completely excluded, as the choice of endometrial preparation protocol was not randomized and may have been influenced by clinician preference or subtle patient characteristics not captured in the dataset. In addition, the findings are applicable only to ovulatory women receiving intensive luteal phase support with combined vaginal progesterone and oral dydrogesterone, and may not be generalizable to populations using alternative progesterone regimens or to anovulatory patients. Finally, serum progesterone concentrations were not systematically analyzed, precluding assessment of whether biochemical luteal adequacy differed between protocols.18
Benefits and Limitations of NC/mNC
Natural and modified natural cycles preserve corpus luteum function, and several large studies suggest comparable or even better outcomes compared with HRT, particularly in ovulatory women. Challenges of true NC include intensive monitoring, risk of premature LH surge, and reduced scheduling flexibility. In anovulatory women, true NC is not feasible. To broaden applicability, ovulation induction (OI) protocols using clomiphene, letrozole, or low-dose gonadotropins are employed, creating hybrid strategies between natural and stimulated cycles.
3. Modified Stimulated Cycles (Letrozole Protocols)
Letrozole, an aromatase inhibitor, has gained prominence as an ovulation induction agent for FET preparation. By increasing endogenous FSH release, it promotes follicular growth while preserving the hypothalamic–pituitary–ovarian axis, ensuring ovulation and corpus luteum formation.
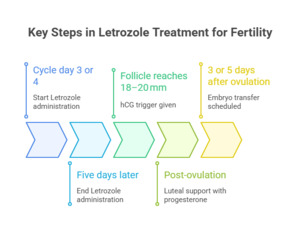
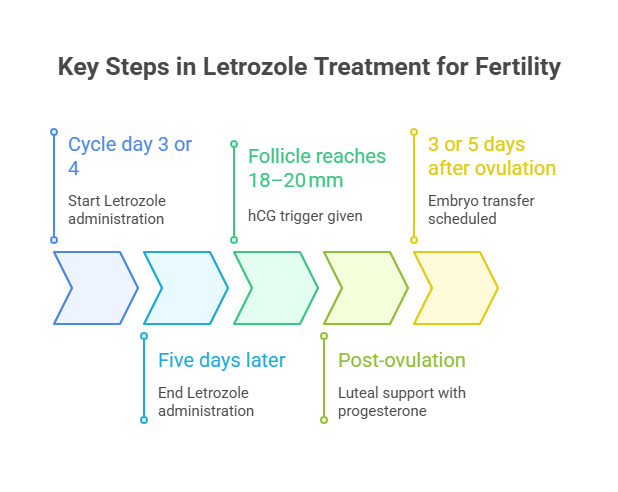
Standard regimens involve administering letrozole 2.5–5 mg daily for five days, typically from cycle day 3 or 4. Follicular growth is assessed by ultrasound, with hCG trigger given at ~18–20 mm. Luteal support is added, and embryo transfer is planned according to developmental stage. (Fig. 3)
Clinical Advantages and Considerations
Beyond inducing ovulation, letrozole exerts favourable endometrial effects. Studies suggest upregulation of receptivity markers such as integrin αvβ3, leukemia inhibitory factor (LIF), and HOXA10/HOXA11 genes, along with enhanced endometrial vascularity. Importantly, systemic estrogen levels remain lower than with gonadotropins, potentially preventing premature endometrial advancement. Letrozole-based stimulation provides an intermediate approach, combining the physiological benefits of natural endometrial preparation with the predictability of controlled cycles. It is generally well tolerated, relatively inexpensive, and when administered in low doses, carries a lower risk of multiple follicular growth compared with gonadotropin stimulation. Nonetheless, close surveillance is necessary to reduce the risk of premature LH surge, and individual variation in endometrial receptivity can still occur, requiring tailored monitoring.
Letrozole protocols have also been evaluated in regularly menstruating women as an alternative to HRT or mNC. In a randomized study, Samsami et al. (2019) found higher, though not statistically significant, pregnancy rates with letrozole compared to HRT.19 Large retrospective studies conducted by Danjun et al. (2021) involving over 6,800 patients, concluded that letrozole-stimulated cycles provide outcomes comparable to natural cycles in ovulatory women, while offering cost-effectiveness and ease of use.20 A meta-analysis including 75,968 FET cycles compared letrozole with other protocols and found comparable clinical pregnancy and live birth rates across letrozole, natural, and artificial cycles, though some subgroups varied. While this pooled analysis supports letrozole as an option, confidence intervals were wide and many individual studies were small, indicating heterogeneity and uncertainty in effect size. This meta-analysis provides the strongest synthesized evidence but also highlights variability and limited precision.21 Cohort evidence suggests letrozole protocols may be associated with higher clinical pregnancy and live birth rates in specific populations (e.g., PCOS), but these are retrospective and not randomized.22 Sample sizes in RCTs are small with insufficient power to detect moderate differences in live birth rates. No large multicentre RCT has definitively established superiority or non-inferiority of letrozole vs other protocols in general FET populations.
The Down-Regulation and Ovulation Induction (DROI) Protocol
The DROI approach integrates pituitary suppression using a GnRH agonist with subsequent ovulation induction, aiming to enhance endometrial receptivity while preserving corpus luteum activity. The protocol typically consists of long-acting GnRHa administration, stimulation with low-dose gonadotropins, ovulation triggering with hCG, luteal phase supplementation, and precisely timed embryo transfer. Early clinical reports suggest higher implantation and clinical pregnancy rates with this method, especially in patients experiencing recurrent implantation failure or suboptimal endometrial development. On a molecular level, improved expression of receptivity markers has also been observed. Despite these encouraging findings, the potential drawbacks include an increased risk of ovarian hyperstimulation syndrome (OHSS), greater financial burden, and the lack of confirmation from large-scale randomized trials.
Mei et al. conducted a large retrospective cohort study of 3030 FET cycles to compare four endometrial preparation strategies: true natural cycle, ovulation induction, HRT, and GnRHa + HRT. Natural-cycle preparation showed superior outcomes, with significantly higher hCG positivity, clinical pregnancy, and live-birth rates compared with the other approaches. GnRHa + HRT also outperformed standard HRT in both clinical pregnancy and live-birth rates. Multivariable analysis highlighted the importance of embryo number, embryo quality, age, BMI, and endometrial thickness in predicting treatment success. Overall, the study suggests that natural-cycle preparation may provide the most favorable reproductive outcomes.23
Cerrillo et al. conducted a prospective trial of 570 FET cycles to compare artificial (HRT) preparation with natural-cycle approaches triggered either by hCG or spontaneous LH surge. Implantation, ongoing pregnancy, and live-birth rates were similar across all protocols, indicating no clear advantage of the natural cycle in overall success. However, miscarriage rates were significantly higher in the HRT group than in both natural-cycle subgroups. Perinatal outcomes were comparable among all methods. The authors note that although success rates are similar, HRT offers practical scheduling benefits and fewer clinic visits, making it a convenient option in high-volume IVF centers.24
4. Adjunctive Strategies
In addition to standard approaches for endometrial preparation, a range of supportive interventions has been investigated to enhance receptivity in difficult clinical scenarios. These adjuncts are particularly considered for women with recurrent implantation failure (RIF), persistently thin endometrium, or uterine conditions that limit successful implantation.
4.1. Platelet-Rich Plasma (PRP) Intrauterine Infusion
Platelet-rich plasma is derived from autologous blood and contains concentrated platelets along with bioactive molecules such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF). Intrauterine PRP infusion has been explored as a means of promoting endometrial proliferation, vascularization, and repair. Several small observational and prospective studies have reported improvements in endometrial thickness and pregnancy outcomes in women with resistant thin endometrium (<7 mm) undergoing FET.25,26
In women with refractory thin endometrium, hysteroscopic injections of PRP led to a clear improvement in endometrial thickness and local blood flow compared with conservative therapy. When PRP was used after standard medical treatment, the gains in endometrial growth were similarly enhanced. The combination of PRP with autologous endometrial cells produced an additional increase in thickness, reflecting stronger stimulation of proliferation and angiogenesis. Overall, PRP alone or augmented with endometrial cells; outperformed conservative management in improving endometrial receptivity.27
However, current evidences remain limited, with considerable heterogeneity in preparation techniques and administration schedules and absence of robust randomized controlled trials. Although the autologous nature of PRP makes it generally safe, its use is still considered experimental and should be restricted to carefully selected cases. Most individual studies on PRP in FET are retrospective observational cohort or case series. Some report endometrial thickness improvement and higher pregnancy rates in women with refractory thin endometrium, but comparisons are often uncontrolled. There are very few RCTs evaluating intrauterine PRP in FET. Heterogeneous preparation methods, dosing, and small sample sizes make effect estimates unreliable. PRP remains preliminary and investigational; benefits are suggested by observational data but not confirmed by high-quality trials.
4.2. Immune Modulation
Immunological mechanisms are increasingly recognized as important determinants of implantation, and dysregulated responses have been linked to some cases of unexplained RIF. Various immune-targeted therapies including low-dose corticosteroids, intralipid infusions, and low molecular weight heparin have been tested as adjuncts during endometrial preparation. The evidence, however, remains inconsistent. while certain subgroups with identified immunologic abnormalities may benefit, randomized trials in broader IVF populations have produced conflicting results. Given the risk of adverse effects associated with systemic immunosuppression, these strategies should be applied cautiously, ideally within clinical trials or highly selected contexts.
4.3. Endometrial Scratching
Endometrial scratching, or deliberate mechanical disruption of the endometrium in the cycle prior to embryo transfer, was once hypothesized to enhance receptivity by triggering an inflammatory and regenerative response. Although early studies suggested potential benefit, more recent high-quality randomized controlled trials, including the large UK “SCRaTCH” study, have failed to demonstrate any improvement in live birth outcomes in unselected IVF patients. As a result, routine use of endometrial scratching is no longer recommended. Its role may be limited to research applications or selected RIF cases where conventional interventions have failed.28
4.4. Other Emerging Approaches
Several other experimental methods are under investigation. These include intrauterine instillation of granulocyte colony-stimulating factor (G-CSF) to encourage endometrial proliferation, and the use of mild ovarian stimulation protocols after GnRHa downregulation in women with endometriosis or adenomyosis. While anecdotal reports and small series have described pregnancies following such interventions, conclusive evidence from well-designed trials is still lacking. At present, these strategies should be regarded as investigational.
Lamee et al. in 2024 investigated the role of endometrial compaction (EC) in predicting outcomes in women undergoing ART and reported that EC was linked to higher clinical pregnancy rates (CPR) and ongoing pregnancy rates (OPR). However, this improvement did not extend to live birth rates (LBR). Endometrial compaction, defined as a reduction in endometrial thickness prior to warmed blastocyst transfer in hormone replacement therapy (HRT) cycles has been associated in earlier studies with enhanced OPR compared to cycles without thickness reduction or with endometrial compaction.29 One retrospective study analyzed 274 hormonally prepared FET cycles to determine whether the endometrium’s response to progesterone specifically, a reduction in thickness from the estrogen phase to embryo transfer—affects pregnancy outcomes. Endometrial measurements at both stages showed that patients whose endometrium compacted by 5%, 10%, 15%, or 20% had consistently higher ongoing pregnancy rates than those with no compaction or an increase in thickness. The likelihood of ongoing pregnancy increased progressively with greater degrees of compaction, demonstrating a clear dose–response relationship. These findings reveal a strong inverse correlation between change in endometrial thickness and pregnancy outcome. Overall, progesterone-induced endometrial compaction appears to be a favorable marker of receptivity in FET cycles.30 One proposed explanation is that failure of compaction may reflect inadequate or resistant progesterone signalling, which could potentially be improved by modifying progesterone supplementation strategy, dosage or its duration. Nevertheless, more recent large-scale analyses have questioned the predictive value of EC, showing no consistent association with outcomes in HRT, modified natural cycle (mNC), or even fresh transfer settings, thereby limiting its reliability as a biomarker. The current evidence are derived predominantly from small, single-centre observational studies, with inconsistent findings and potential confounding, and no adequately powered randomized controlled trials have directly evaluated compaction as an independent predictor or therapeutic target. Consequently, endometrial compaction should be regarded as a hypothesis-generating observation rather than an established clinical marker, pending validation in large prospective studies.
Clinical Protocol Selection and Practical Recommendations
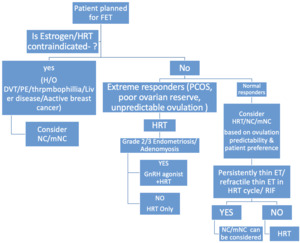
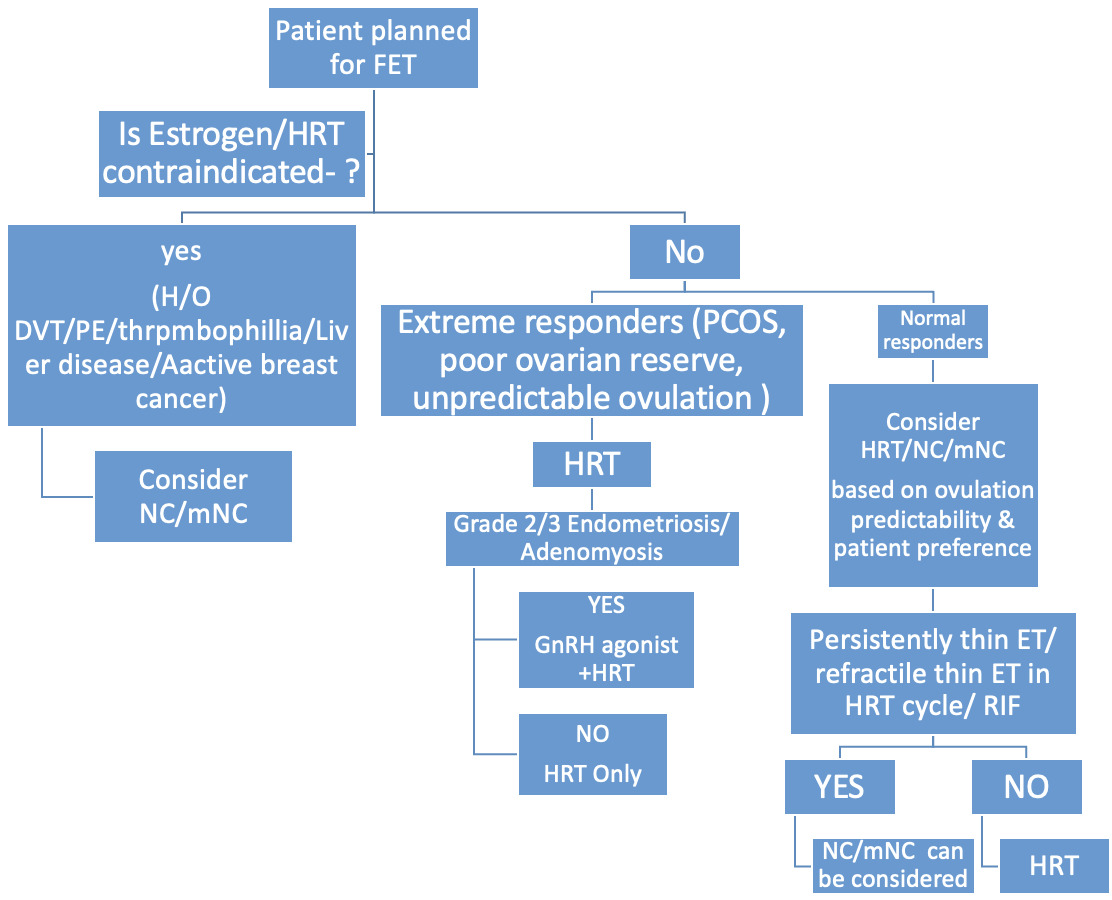
Selecting the most suitable endometrial preparation strategy should be individualized, taking into account the woman’s ovulatory function, prior reproductive outcomes, uterine condition, associated medical issues, practical considerations such as monitoring or travel plans and her own treatment preferences. (Fig. 4)
Future Directions
Overall, current evidences do not support any single endometrial preparation method as definitively superior for women undergoing frozen embryo transfers or fresh donor-oocyte cycles. The available trials, most of which provide low quality evidences, suggest that stimulated FET cycles may offer a modest improvement in clinical pregnancy compared with programmed cycles, while natural cycles appear to lower the risk of cycle cancellation. The benefit of adding GnRH-agonist pre-treatment remains uncertain, although one small study suggests a possible improvement in live births; however, GnRH-antagonist protocols may perform better in terms of clinical pregnancy rates than agonist-based regimens. In donor-oocyte cycles, pregnancy and cancellation outcomes seem more favorable when progesterone is started on or just after the donor’s oocyte retrieval. Overall, larger, well-designed RCTs are needed to determine the most effective and reliable strategies for endometrial preparation.31
Current evidence suggests that natural or modified natural cycles may offer better outcomes than hormone-replacement protocols for FET, although overall data quality remains low. Optimal timing of progesterone exposure and recognition of individual variations in serum progesterone are emerging as critical factors influencing implantation success. Future research will likely focus on refining timing strategies and evaluating progesterone-rescue approaches for women with low pre-transfer levels.32 The refinement of endometrial preparation protocols for FET is ongoing, with several important areas of investigation likely to guide progress in the coming years.
Large, Multi-Center Randomized Trials
Much of the current evidences supporting newer approaches such as the DROI protocol or intrauterine PRP infusion originate from retrospective analyses or single-centre studies. To establish clinical validity, robust multi-centre randomized controlled trials are needed, directly comparing letrozole - based regimens , DROI, natural cycles, and HRT in well-defined populations, including those with PCOS, endometriosis, and recurrent implantation failure.
Long-Term Obstetric and Child Health Outcomes
Although many studies report short-term implantation and pregnancy rates, relatively few assess long -term maternal complications, perinatal outcomes, or child health beyond delivery. Future research should emphasize longitudinal follow-up to determine whether different endometrial preparation strategies exert lasting effects on maternal and offspring well-being.
Molecular Profiling and Personalized Medicine
Advances in transcriptomic and proteomic technologies, along with tools such as endometrial receptivity assays, open the possibility of tailoring the protocols as per individual’s biological profiles. Integrating these molecular diagnostics into clinical care may allow alignment of protocol choice with each patient’s unique implantation window and endometrial signature.
Standardization of Adjunctive Treatments
Emerging adjuncts including PRP, granulocyte colony-stimulating factor (G-CSF), and immune modulators require harmonization of preparation methods, dosing, and timing. Standardized protocols will facilitate reproducibility across centres and enable meaningful meta-analyses.
Incorporation of Patient-Reported Outcomes
Future studies should move beyond clinical endpoints and include patient-reported outcomes such as treatment satisfaction, physical and emotional burden, and time-to-pregnancy. These measures are vital to evaluate the true impact of different strategies in real-world practice.
Conclusion
Endometrial preparation remains central to the success of frozen embryo transfer cycles. Conventional approaches—HRT and natural cycles—have long provided reliable outcomes, yet newer strategies offer the potential to further optimize receptivity, enhance IVF success, and improve maternal safety in selected groups. Adjunctive therapies, including PRP and immune modulation, continue to be experimental but may represent promising options for women with limited alternatives. Individualization of frozen embryo transfer protocols is likely more clinically relevant than identifying a universally superior approach between hormone replacement therapy and natural cycle regimens. Increasing evidence suggests that the absence of corpus luteum activity in artificial cycles may be associated with a higher risk of hypertensive disorders of pregnancy and other adverse obstetric outcomes. Furthermore, several adjunctive interventions, including platelet-rich plasma infusion, immunomodulatory therapies, and other so-called “add-ons,” currently lack robust high-quality evidence and should therefore be regarded as investigational rather than components of routine clinical practice. Ultimately, selecting the most appropriate protocol must be individualized, balancing clinical evidences with patient characteristics, safety considerations, and practical constraints. However, significant gaps persist regarding long-term maternal and neonatal outcomes across different preparation strategies, and high-quality randomized controlled trials remain limited. Addressing these evidence gaps is crucial for developing truly evidence-based, patient-tailored pathways. The optimal protocol will be one that not only maximizes live birth rates but also optimises maternal and neonatal health, minimizes treatment complexity, and aligns with patient values and preferences.
Use of Artificial Intelligence
Artificial intelligence (AI) tools were used only to support language refinement and organization of the manuscript. No AI tool was used for data generation, analysis, interpretation or creation of scientific content. All clinical concepts, evidence synthesis, and conclusions were developed and verified entirely by the authors. The authors take full responsibility for the accuracy and integrity of the manuscript.
Ethics approval and consent to participate
This manuscript is a narrative review and does not involve any primary research on human participants or animals. Therefore, approval from an institutional ethics committee and informed consent were not required. The review was conducted in accordance with the principles of the International Committee of Medical Journal Editors (ICMJE) and the Committee on Publication Ethics (COPE).
Competing Interests
No competing interests were disclosed
Funding statement
This review received no specific grant or financial support from any funding agency, commercial entity, or not-for-profit organization.