Highlights
-
AMH levels decrease as women age advances, reflecting a decline in their ovarian reserve.
-
Measuring AMH levels can help assess a woman’s fertility potential, diagnose certain reproductive conditions such as PCOS and decreased ovarian reserve, and research reproductive health.
-
It is important to interpret AMH levels in the context of an individual’s unique circumstances, as levels can vary greatly between individuals and can also be affected by other factors, such as PCOS.
-
Understanding the relationship between age and AMH levels is crucial for making informed decisions about family planning and fertility treatments.
Introduction
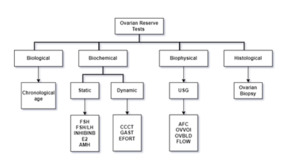
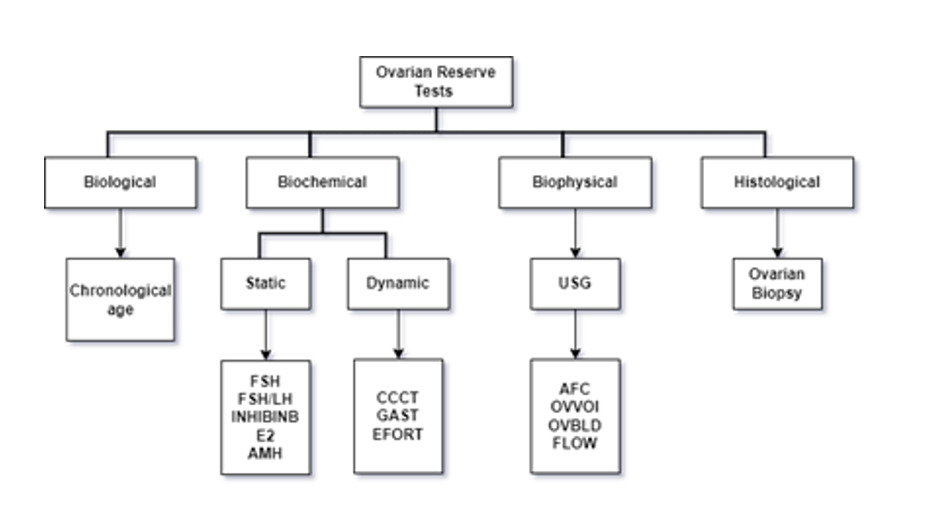
The ovarian reserve is related to the resting primordial follicle pool size, which is consistently declining with advanced reproductive age.1 Different techniques are established to measure ovarian reserve (Figure 1) (remaining follicular pool) that may be categorized as - biological, biochemical, biophysical, and histological tests.2 Chronological aging is a low acceptable technique for ovarian reserve. Anti-Mullerian hormone (AMH) test and AFC are acceptable and widely used in routine practice.3
Anti-Mullerian hormone AMH) is a homodimeric glycopeptide in reproductive-aged females and is predominantly derived from granulosa cells in the ovary.4,5 AMH expression is highest in secondary, preantral, and small antral follicles up until approximately 4 mm in size, and it stops being expressed by granulosa cells when follicles measure in the 4 to 8 mm range.6 Anti-Müllerian hormone (AMH) is a protein hormone produced by the developing follicles in the ovaries. Its main function is to inhibit the development of female reproductive organs in fetuses during pregnancy. AMH levels are relatively stable throughout the menstrual cycle. The levels of AMH can help doctors determine a woman’s fertility potential and can be useful in diagnosing conditions such as polycystic ovary syndrome (PCOS) and premature ovarian failure. AMH testing is becoming more common to predict a woman’s response to fertility treatments such as in vitro fertilization (IVF). Women with higher AMH levels tend to respond better to ovarian stimulation and produce more eggs during IVF cycles.7
Since AMH is produced in small ovarian follicles, blood levels of AMH is used to measure the size of the pool of growing follicles in females.8 Research shows that the size of the growing follicle pool is heavily influenced by the size of the remaining primordial follicles.9 With an increase in female age, the size of the pool of the remaining microscopic follicles decreases.10,11 AMH level starts a log-linear decline approximately 15 years before menopause and drops to a very low level before menopause.12 Instructively, the physiology of female fertility explains the diminishing number and quality of oocytes with increasing age.
In recent years, predicting future reproductive potential has become crucial because of lifestyle, social, and cultural factors, which delay the child’s bearing. So, AMH levels can be used for the evaluation of infertility to guide clinicians in making appropriate decisions regarding the management of women seeking conception.13 The studies collectively suggest that Indian women, whether seeking infertility treatment or with a history of natural conception, experience a more rapid decline in AMH levels and have significantly lower AMH concentrations compared to Caucasian women14 This highlights the importance of early AMH testing to assess ovarian reserve in Indian women and emphasizes the need for tailored approaches in fertility management and treatments for this population.15–17
The current literature18–20 lacks sufficient data on the cut-off value of AMH levels in Indian infertile females.
This attempts to showcase the large data on the Indian population on such a large scale, representing different geographical areas. Different transformation and regression models are compared for producing efficient nomograms. This would support the utilization of cut-off for Anti-Mullerian Hormone decline over age. The present study aimed to find the age-specific variation of AMH for the Indian population in different geographical areas in India and produce different nomograms through regression models for year-wise trends in AMH level in Indian women.
Method
Study Design
This retrospective analysis was conducted at 101 centers of IIHPL. Data was collected from August 2015 –August 2022.
Study Population
The study included 1,71,595 females aged 21-60 years who underwent IVF treatment at IIHPL clinics. The inclusion criteria were broad, encompassing all women seeking treatment during the study period. The missing data was not considered for the analysis. The AMH measurement was done using Cobase411 as the tests were done in a single chain of laboratories to ensure consistency in results. The confounding factors affecting AMH are not considered in this study.
Blood Collection and AMH Analysis
Venipuncture was performed to collect 5 mL of blood. The Cobas e411 AMH immunoassay was used to determine AMH levels. The standard procedure of IIHPL was used for collecting serum samples in which the samples were kept at room temperature for 30-60 min to permit full clotting. The samples are centrifuged for 15 min at 2200-2500 revolutions per minute (rpm) to separate the serum from cells and other blood components. The serum was transferred to a labeled plastic screw-cap vial. Cobas e411 AMH immunoassay uses two AMH–specific antibodies in a sandwich format to determine the serum hormone level.
Ethical Clearance
The study received approval from the IIHPL Institutional Ethics Committee, ensuring adherence to ethical guidelines and patient confidentiality.
Outcomes
This retrospective multicenter study provides valuable insights into the geographical distribution of AMH levels of women consulting for infertility management at tertiary care centers across India. The results may aid in predicting IVF success and optimizing treatment strategies for infertile couples.
Statistical Analysis
Data Collection and Preprocessing
Data for the study were retrieved, collected, and tabulated from the central electronic database system. Women’s data with missing age or AMH levels were excluded from the analysis. They added to the list of limitations for such a huge sample size. Due to limited accessibility, it was not possible to consider all co-variates. Due to limited accessibility, it was not possible to consider all the covariates for such a huge sample size.
Data Transformation
Since the distribution of AMH levels was non-Gaussian with positive skewness, parametric methods were employed for data transformation. Different transformation methods were applied to the AMH dataset, including logarithmic, square root, and power transformations (0.3 & 0.4). Regression models were then used to estimate the required parameters that best fit the model. Power transformation was carried out stepwise from 0.1 to 0.9 with an increment of 0.1 power.
Model Fitting
Based on the Normal Q-Q plot analysis, power transformations of 0.3 and 0.4 demonstrated the longest linear segments, indicating the most approximate Gaussian distribution(Table 1). These transformations were selected for model fitting (Figure 4).
Evaluation Metrics
The coefficient of determination (R2) and adjusted R2 (R2 adj) were calculated to assess the goodness of fit. Models with higher R2 and R2 adj values and the lowest AFC (Akaike’s Final Criterion) were considered the most desirable. The best model selection was based on the trade-off between goodness of fit and model complexity.
Nomogram Construction
For the nomogram construction, the AMH dataset was power transformed using various values of ‘p’ (power transformation) ranging from 1.0 to 0.0 with a decrement of 0.1. The optimal ‘p-value (p0)’ was empirically determined to be 0.4, resulting in the most extended linear segment in probability, approximating a Gaussian distribution. All p-values less than 0.05 were considered statistically significant. Statistical analysis was conducted using the Statistical Package for Social Science (IBM SPSS), version 28.0.
Results
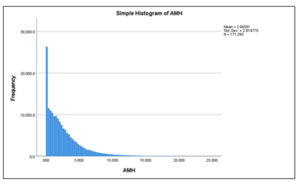
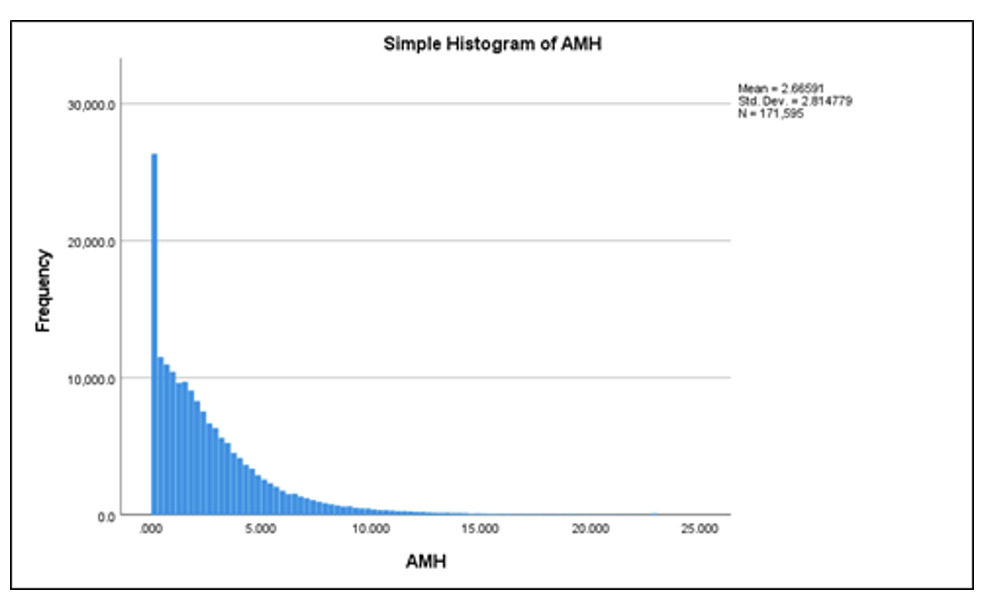
The study included 171,595 women who opted for IVF treatment at different Indira IVF Hospital Private Limited (IIHPL) centers across India between August 2015 and August 2022 (Figure 2). The age range of the participants was 21 to 60 years. Data analysis compared the Anti-Mullerian Hormone (AMH) levels in different age groups and geographic regions. The variation in AMH levels was shown using a histogram in Figure 3.
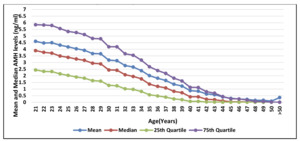
The results revealed a significant correlation between age and AMH levels. The AMH levels decreased with age, with the highest median AMH level observed in women below 25 years (4.42 ng/mL) and the lowest in women aged 50 years or above (0.24 ng/mL).
A model-fitting analysis was conducted to find the best transformation for normalizing the relationship between age and AMH. The power transformations of 0.3 and 0.4 showed the most extended linear segment in the Normal Q-Q plot (figures 4-6), indicating their effectiveness in the model. The linear regression model with the power transformation of 0.4 (AMH0.4 = a + b*Age) had an R-squared value of 0.290, demonstrating a good fit for the data.
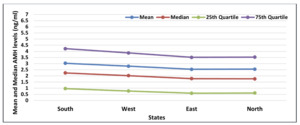
Furthermore, the study examined the AMH levels in different states and zones across India (Table 4). The results showed variations in AMH levels among different states, with Haryana and Karnataka having relatively higher AMH levels than other states. Similarly, the South zone had higher median AMH levels than other zones (Figure 8).
This study provided valuable insights into the relationship between age and AMH levels in women opting for IVF treatment in India. The findings emphasize the importance of considering age-related variations in AMH levels during fertility assessments and treatment planning. The identified model with the power transformation of 0.4 offers a reliable approach for predicting AMH levels based on age (Figure 7-9). These results contribute to a better understanding of ovarian reserve and can aid in optimizing infertility treatment strategies.
Comparing the study results with other relevant studies, AMH levels showed consistent trends with age in various geographic regions and populations. For instance, studies conducted in the United Kingdom, Korea, and Egypt also reported a decline in AMH levels with increasing age.18–20
The study’s strength lies in its large sample size and the multicenter approach, providing comprehensive data from different regions of India (Figure 8). However, certain limitations should be acknowledged, such as the retrospective nature of the study and the study could not address the migrating population.
This study comprehensively analyzes AMH levels in women undergoing IVF treatment in India. The results highlight the impact of age on AMH levels and provide valuable insights into the relationship between age and ovarian reserve. The identified power transformation model can be a valuable tool for predicting AMH levels based on age. These findings have significant implications for fertility assessment and treatment strategies, enabling more personalized and effective infertility management.
Discussion
The widespread adoption of Assisted Reproductive Technology (ART) has brought to light the issue of poor ovarian reserve in India, with approximately 10% of women undergoing in-vitro fertilization experiencing suboptimal responses to gonadotropin stimulation.18,21 Anti-Mullerian hormone (AMH) has emerged as a critical biomarker associated with ovarian reserve. Low levels in younger age groups raise concerns, necessitating appropriate guidance and management.22 Hence, it becomes imperative to investigate the correlation between age and AMH levels in Indian females and forecast the geographical distribution of AMH levels to facilitate effective fertility counseling and management.23
Our study examined the relationship between age and AMH levels using a vast dataset comprising 171,595 apparently unhealthy Indian females who sought care at Indira IVF Hospital Private Limited (IIHPL) between August 2015 and March 2022. Employing the power transformation technique for AMH normalization, we explored various regression models to identify the best fit. Our findings revealed that age alone contributes to about one-third of the variation in AMH levels, a significant finding demonstrated by the quadratic model of log AMH.
A retrospective study(n=54,473) was conducted15 on Indian infertile females to showcase the prevalence of low ovarian reserve in Indian women and compare age-specific AMH levels in Indian controls and Caucasian women. The distribution of median AMH values and percentage fall in different age groups was considered. Their study concluded that Indian women, whether seeking infertility treatment or with a history of natural conception, experience a faster decline in AMH as compared to their Caucasian counterparts.
An Indian laboratory conducted16 a retrospective study(n= 219,227) to find a trend between AMH and age to identify the cut-off to assess a significant drop in AMH levels in PAN India. They showcased the nomogram using mean and median levels of AMH levels across the Indian population which highlighted the requirement of AMH testing before 27 years of age to monitor ovarian reserve.
A recent prospective cohort study (n=2758)17 compared a healthy European (Netherlands, Belgium, Germany, France, and Turkey) cohort and healthy Indian women (Kolhapur) and women visiting fertility clinics India (Kolhapur). Healthy participants were recruited from January 2016 to July 2020. All participants had regular mensuration cycles aged between 20 and 45. The median and untransformed data were presented in descriptive and log-transformed forms. They concluded that healthy Indian women have significantly lower AMH concentrations at all ages than European women. Irrespective of race there is an AMH decline in both European and Indian healthy women.
Consistent with prior research,24 we observed a linear decline in AMH levels with reproductive age.24 However, some studies have suggested non-linear declines in AMH levels.18,21 Some have explored age-related AMH levels specific to Egyptian females in a multistep scheme using power transformation.25 Although our study did not validate the predictive model using training and testing data, this could be an avenue for future research.
A similar prospective study conducted by Lee et al.26 evaluated AMH variation with age among infertile patients. Their study reported an overall mean AMH level of 4.09 ± 3.71 ng/mL, with a progressive decline as age increased. In contrast, our study, based on more recent data (August 2015 –August 2022) showed a lower mean AMH level of 2.66 ± 1.89 ng/mL, indicating a steep decline in AMH levels with age.
El-Attar et al.25 conducted a study at a University Hospital in Egypt, where they developed a nomogram for 998 apparently healthy females using the electroluminescence technique to measure AMH levels. They employed the Box-Cox transformation to achieve a Gaussian distribution of AMH for deriving reference intervals.
Likewise, in our study, we also constructed a nomogram and applied power transformation with three regression models: Linear, Quadratic, and Cubic. We employed various models to identify the best-fit model that ensured the proper distribution of AMH levels in our dataset. This approach allowed us to develop a reliable AMH nomogram that could assist in counseling infertile patients and guiding treatment options effectively. The study conducted at a specified fertility center in Ghana27 investigated the relationship between age and Anti-Mullerian hormone (AMH) levels in 426 women. The mean age of the participants was 35.25±6.33 years, and the mean AMH level was 2.8±2.6 ng/mL. The results revealed a significant negative correlation between age and AMH levels, indicating that younger women had higher AMH levels than older women. None of the younger women had AMH levels below 0.30 ng/mL. This study provides valuable insights into AMH variation with age in the Ghanaian population and its implications for fertility counseling and management.
The state-wise and zone-wise variation in AMH levels yielded insightful results. The South zone exhibited the highest AMH levels, whereas the East zone had the lowest, with Assam being the state with the lowest AMH levels. This geographical variation may be attributed to diverse factors such as lifestyle, genetics, and environmental influences. The implications of our study are highly relevant for Indian infertile females seeking fertility counseling for delayed pregnancy or marriage. The observed decline in AMH levels with age significantly affects reproductive health and family planning decisions. The AMH nomogram developed in our study can serve as a valuable reference tool for counseling infertile patients and guiding treatment options.
However, we acknowledge certain limitations in our study, being hospital-based, which may limit its generalizability to the broader population. Future research could explore additional factors such as body mass index, lifestyle, and conditions like polycystic ovary syndrome (PCOS) to understand AMH levels in Indian females comprehensively. Considering the retrospective nature of the study, we had no control of the data; however, the data from the laboratory, hence the margins of error must be low. The study is not immune to sampling bias as its data was collected from apparently sub-fertile women only. However, considering the pan-India presence of Indira IVF and its huge sample size to has a lesser impact on the results.
The study’s strength lies in its extensive sample size, which boosts its statistical power and ensures that the findings are more representative of the target population. Although the study is hospital-based, the large sample size and comprehensive analysis contribute to its potential generalizability to a broader population of infertile females in India. The study’s findings have important clinical implications for fertility counseling and management, particularly in guiding family planning decisions and addressing concerns related to ovarian reserve and AMH levels. Overall, the combination of large sample size, long study duration, standardized measurement methods, advanced statistical analysis, and valuable clinical implications makes this research study a robust and significant contribution to India’s reproductive medicine and fertility counseling field.
Our study is one of the largest and most comprehensive analyses of AMH levels among infertile Indian females. The observed steep decline in AMH levels with age underscores the critical role of AMH as a biomarker in fertility counseling and management. Our findings contribute valuable insights into the understanding of ovarian reserve in the Indian population and have potential implications for improving fertility treatments and family planning decisions. The study is about the prevalence and geographical distribution concerning ART outcomes, specifically not biomarkers.
Future studies should explore additional factors and validate predictive models to enhance further our understanding of AMH variation and its impact on fertility outcomes.
Conclusion
Understanding the relationship between age and AMH levels is crucial for assessing fertility potential, diagnosing reproductive conditions, and researching reproductive health. This study presents the age-specific Anti-Mullerian Hormone (AMH) levels for women accessing IVF services in IIHPL India. It is highly crucial that females with AMH levels <0.8ng/mL require proper counseling and adequate IVF treatment. such patients are known to respond poorly to stimulation.
Conflict of Interest
No conflict of interest exists among authors.
Funding
There is no funding.
Informed Consent Form
Not Applicable
Data availability Statement
The dataset for this study will be made available on request.
Acknowledgments
We want to acknowledge the Research and Development Team, MIS Team, and others who have contributed to providing accurate data. The authors especially thank Centre Heads for their full support in this study. The authors are also grateful to all the women whose data was part of the study.
Authors’ Contribution per CRediT
Conceptualization: Kshitiz Murdia; Data curation: Vipin Chandra; Formal analysis: Nihar Ranjan Bhoi; Funding acquisition: NA; Investigation: NA; Methodology: Nihar Ranjan Bhoi; Project administration: Vipin Chandra; Resources: Naval Shah, Ritesh Aggarwal, Vipin Chnadra; Software: Nihar Ranjan Bhoi; Supervision: Nitiz Murdia, Kshitiz Murdia; Validation: NA; Visualiztion: Nitiz Murdia; Writing – original draft: Isha Suwalka; Writing – review and editing: Dr Nagadeepti Naik , Dr. Shipra Nigam , Dr. Ritu Puhani.



_before_the_transformation.png)




_before_the_transformation.png)