Introduction
Rekovelle® (follitropin delta) is a novel recombinant human FSH (rFSH) expressed from a host cell line of human fetal retinal origin (PER.C6®) and is the first commercially available rFSH product derived from human cell lines,1,2 which allows the individualization of the initial dose of gonadotropin using predictive response factors COS, such as anti-müllerian hormone (AMH) levels and body weight.3
The study entitled “The Evidence-based Stimulation Trial with Human rFSH in Europe and Rest of World (ESTHER-1)” was a noninferiority, randomized clinical trial aiming at comparing an individualized fixed dose of follitropin delta determined by an algorithm based upon each woman’s AMH and body weight with a conventional adjustable follitropin alfa dose in patients undergoing their first in vitro fertilization (IVF) treatment. This multicenter study showed that continued pregnancy implantation and live birth rates were similar in patients treated with follitropin delta and conventional follitropin alfa.4
Usually, the individual initial daily dose of Rekovelle® is determined based on the patient’s serum AMH value, obtained using the Roche Elecsys® AMH Plus immunoassay, and body weight in kg. In clinical practice, different approaches can be adopted to obtain the most satisfactory ovarian response to stimulation. In our center, the observation that previous patients undergoing COS with a dose of Rekovelle® calculated according to the drug leaflet had an ovarian response below the doctors’ expectations, represented by a low number of eggs retrieved and oocyte maturity rate, led to the use of a fixed daily dose of 16 mcg, regardless of AMH value or body weight. To date, no descriptive studies are addressing different approaches on Rekovelle® use. This study aimed to describe data on “real-world” Rekovelle® administration concerning the response to COS, embryo quality, and clinical ICSI outcomes. Two other cohorts of patients (intern and extern) were added as references: the population included in the ESTHER-1 trial (extern), and a population undergoing COS with follitropin alpha (on site), starting with 300 IU, applying the equivalence factor between delta and alpha follitropins previously described.5
Materials and Methods
Study design
This non-interventional study based on secondary use of data included patients undergoing ICSI treatment in a private university-affiliated IVF center from January 2018 to December 2021. To be eligible for inclusion in this study, each patient or cycle had to meet the following inclusion criteria: pre-menopausal women, undergoing COS with Rekovelle® (16 ug) daily for ICSI; diagnosed with infertility, or with partners diagnosed with male infertility factors, eligible for ICSI using fresh sperm from partner ejaculation; presenting with both ovaries.
Ovarian response to stimulation and laboratorial and clinical outcomes of 362 ICSI cycles matching de inclusion criteria were described. The primary outcome measures were the numbers of retrieved oocytes and maturity rates, and the secondary outcome were the ongoing pregnancy rates per fresh embryo transfer and per fresh and/or frozen-thawed embryo transfer. Morphokinetic parameters were also extracted and described for embryos that were cultured in a time lapse imaging incubator. As reference, morphokinetic parameters from implanted embryos reported in previous studies were also described. The results obtained with the population enrolled in the ESTHER-1 trial (extern), and a population undergoing COS with follitropin alpha (on site), starting with 300 IU were also described as references. Bearing in mind that this is a descriptive and non-comparative study, we only described the mentioned parameters.
Controlled ovarian stimulation and follicular aspiration
The stimulus was started with the administration of 16ug Rekovelle®. On the sixth day of stimulation, the gonadotropin dose was adjusted if necessary, and maintained until the day of hCG trigger.
In the follitropin alpha reference group, the stimulus was started with the administration of 300 IU r-FSH (GONAL-f, Merck KGaA, Darmstadt, Germany). On the sixth day of stimulation, the gonadotropin dose was adjusted if necessary and maintained until the day of trigger.
Pituitary block for both protocols was performed by administering the Gonadotropin Releasing Hormone antagonist analog (GnRH, Cetrotide®, Merck KGaA, Darmstadt, Germany), when at least two follicles with a diameter ≥ 14mm were visualized. The administration of recombinant human Chorionic Gonadotropin (r-hCG, Ovidrel®, Merck KGaA, Darmstadt, Germany) subcutaneously (SC), used to induce final follicular maturation and luteinization, was performed when at least three follicles reached ≥ 17mm in diameter. Whenever the patient was at risk of developing OHSS, GnRH analog (0,2 mg triptorelin acetate, Gonapeptyl daily®, Ferring GmbH, Kiel, Germany) was administered SC instead of r-hCG. Oocyte recovery was performed 37 hours after administering r-hCG or GnRH analog through transvaginal follicular aspiration guided by ultrasound, the patient being subjected to sedation. Intracytoplasmic sperm injection (ICSI) was performed in all mature oocytes.
Embryo culture
The embryos were cultured in a 50 µL drop of medium (Global®; LifeGlobal, CT, USA) covered with paraffin oil in a humidified atmosphere under 5% O2 and 6% CO2 at 37°C for five days, until the fifth day of embryo development.
For embryos that were cultured in a time lapse imaging incubator, injected oocytes were individually cultured in a 16 well culture dish (Embryoslide, Unisense Fertilitech, Aarhus, Denmark), filled with 360 μl of continuous single-culture media (Global® total®, LifeGlobal) and overlaid with 1.8 ml of mineral oil (Paraffin oil P.G., LifeGlobal), until day five of embryo development. The TL-monitored incubator (EmbryoScope+, Unisense Fertilitech, Aarhus, Denmark) was set at 37 °C, 6% O2 and 7.2% CO2. Embryos were photographed every 10 minutes, in eleven focal planes. Timings to pronuclei appearance (tPNa) and fading (tPNf), to two, three, four, five, six, seven, and eight cells (t2, t3, t4, t5, t6, t7, and t8, respectively), to morulation (tM), start of blastulation (tSB) and to blastulation (tB) were recorded. Durations of cell cycles (second, cc2, t3–t2 and third, cc3 t5–t3), and timing to complete synchronous divisions s1 (t2–tPNf), s2 (t4–t3), and s3 (t8–t5) were also calculated.
Clinical Follow-up
Embryo transfers were performed on day 5 of embryonic development and one or two embryos were transferred per patient depending on maternal age and embryo quality. Surplus high-quality embryos were cryopreserved.
Women with a positive serum βhCG test, performed 10 days after the embryo transfer and repeated two days later, had a transvaginal ultrasound scan 2 weeks later. Clinical pregnancy was diagnosed after fetal heartbeat was detected. The clinical pregnancy rate was calculated per transfer. Spontaneous abortion was defined as clinical pregnancy loss before 20 weeks. Continued pregnancy was defined when the pregnancy completed ≥20 weeks of gestation. Spontaneous abortion rate was calculated per clinical pregnancy. Ovarian hyperstimulation syndrome (OHSS) rate was calculated per the number of patients undergoing COS.
Statistical analysis
No statistical hypothesis was pre-specified since it is a descriptive study. This study employed a single-cohort, open-label design with a synthetic control as a reference (665 patients enrolled. The primary and secondary outcomes were described using descriptive statistics (means, standard deviations [SD], or frequencies, and percentages, whichever are applicable). Morphokinetic parameters recorded for embryos that were cultured in a time lapse imaging incubator were described, as well as those from implanted embryos reported in the studies from Herrero, Tejera6 and Meseguer, Herrero.7
Descriptive analysis was performed using the Statistical Package for the Social Sciences (SPSS) Statistics 21 (IBM, New York, New York, USA).
Results
A total of 362 ICSI patients/cycles in which follitropin delta was used for COS were included in this study. The mean female age was 37.3 ± 3.1 years. A total of 86 women (23.8%) underwent fresh embryo transfer, of whom 52 (60.5%) had day-5 embryo transfer, and 34 had day-3 embryo transfer, resulting in 22 ongoing clinical pregnancies (25.6%). A total of 20 (5.5%) light/moderate OHSS cases (recommendation of general care at home, no hospitalization required) were reported. Preimplantation genetic testing for aneuploidy (PGT-A) was performed in 88 cycles, with a 38.8% euploidy rate (Table 1). One hundred and sixty-six patients achieved pregnancy following fresh or frozen-thawed embryo transfer, resulting in a cumulative clinical pregnancy rate of 45.9%.
Figure 1 illustrates a comparison between the starting doses of Rekovelle® in the modified protocol and how much the dose would be if the protocol were followed according to the Rekovelle® label.
Patients were divided into three subgroups according to maternal age: ≤ 35y-old, between 36 and 39 y-old, and ≥40y-old, and into four subgroups according to maternal BMI: < 18.5, between 18.5 and 24.9, between 25.0 and 29.9, and ≥ 30. Demographic characteristics, ovarian response to COS, laboratory and ICSI outcomes for subgroups of maternal age and BMI are given in Tables 2 and 3 and Figures 2 and 3, respectively.
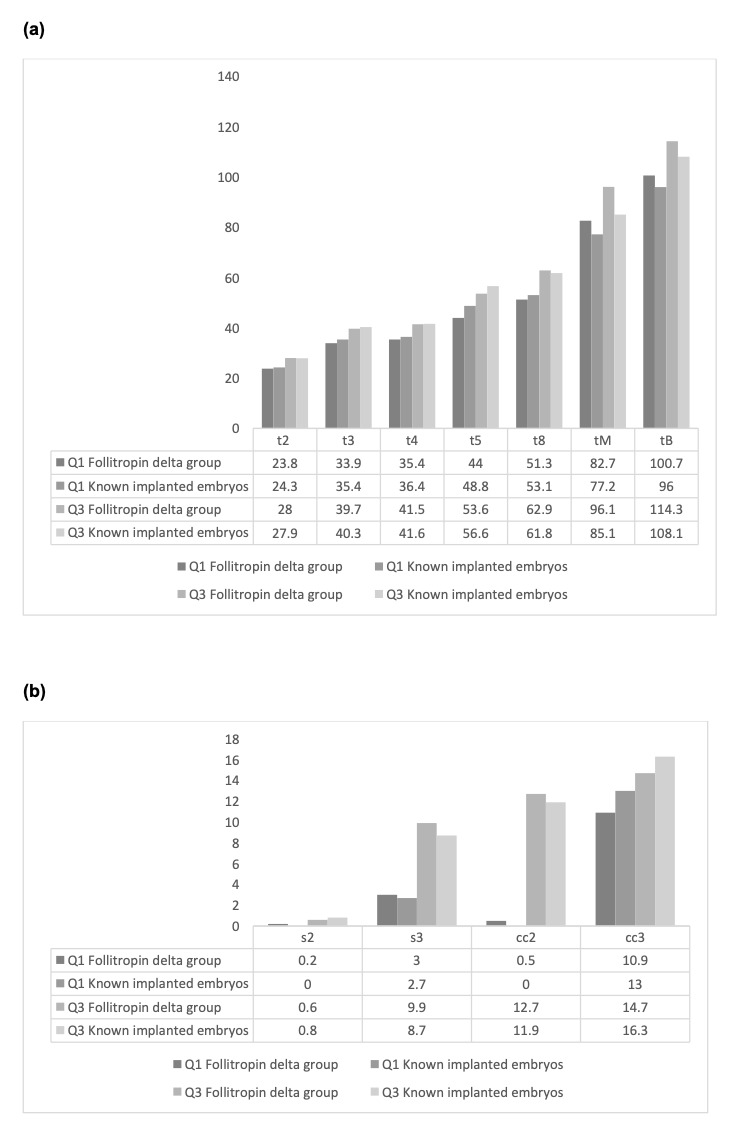
Description of morphokinetic parameters recorded from 1206 embryos originating from 194 patients and those from implanted embryos reported in the studies by Herrero et al.6 and Meseguer et al.7 is illustrated in Figure 4.
In the ESTHER-1 trial, 665 patients were treated with personalized doses of follitropin alpha. Mean female age was 33.4 ± 3.9 years. All women underwent fresh embryo transfer on day-5 embryo, resulting in 211 ongoing clinical pregnancies (31.7%). A total of 23 (3.5%) of any grade OHSS were reported, of those 2 were severe grades (0.3%) and required hospitalization (Table 4).
An intern cohort of follitropin alpha-stimulated patients was also used as a reference (401 patients/cycles). The mean female age was 37.5 ± 3.2 years. A total of 105 women (26.2%) underwent embryo fresh embryo transfer, of whom 60 (57.1%) had day-5 embryo transfer, and 45 had day-3 embryo transfer, resulting in 26 ongoing clinical pregnancies (24.8%). 19 (4.7%) light/moderate OHSS cases (recommendation of general care at home, no hospitalization required) were reported. Preimplantation genetic testing for aneuploidy (PGT-A) was performed in 121 cycles from the Follitropin alpha group, with a 42.9% euploidy rate. One hundred eighty-five patients achieved pregnancy following fresh or frozen-thawed embryo transfer, resulting in a cumulative clinical pregnancy rate of 46.1% in the Follitropin alpha group (Table 5).
Discussion
In this study, we aimed to describe data on “real-world” Rekovelle® administration with respect to the response to COS, embryo quality, and clinical ICSI outcomes. What justified the change in protocol was the observation that previous patients undergoing COS with a dose of Rekovelle® calculated according to the drug leaflet had an ovarian response below the doctors’ expectations, represented by a low number of eggs retrieved and oocyte maturity rate. The main purpose of modifying the protocol was to increase the number of eggs retrieved and the rate of oocyte maturity, which would most likely result in more embryos available for transfer and freezing, with the ultimate objective of increasing the cumulative clinical pregnancy rate.
We observed that patients in the Follitropin delta group showed acceptable outcomes regarding number of follicles and oocytes, oocyte maturity rate, blastocyst development and clinical outcomes. The evaluated outcomes were considered satisfactory in patients in different age groups and in different subgroups of BMI. Figure 2 clearly illustrates that similar mean total doses of follitropin delta were administered among the three subgroups of female age, resulting in satisfactory rates that are different between the three groups as they are affected by maternal age. Figure 3 shows a similar result for the patients, but this time in BMI subgroups.
We also observed that embryos deriving from women in which follitropin delta was used for COS when cultured in a time-lapse imaging incubator showed morphokinetic development that seemed equivalent to those from implanted embryos reported in previous studies, demonstrating the high implantation potential of those embryos. This was further corroborated by satisfactory cumulative implantation rates observed for this group of patients. Results from PGT-A analysis suggest that the modified protocol does not interfere with oocyte and embryo competence since euploidy rates were also within the expected ranges.
Results obtained in the ESTHER-1 trial were also described. The mean age of patients in the ESTHER-1 trial was much lower than in our cohort, but the number of eggs retrieved appears similar. The ESTHER-1 study did not report an oocyte maturity rate. Still, due to the experience of participating in the trial and the very low fertilization rate reported in the ESTHER-1 trial, the maturity rate was likely much lower than expected. This is reflected in a lower number of blastocysts obtained and consequently frozen, which were also much lower than in our cohort of patients with a much higher mean maternal age. The better clinical rates reported in the ESTHER-1 study are justified by the lower mean age of the patients when contrasted with those of our cohort. In addition, eligibility criteria for the ESTHER-1 trial were much stricter than those from our descriptive study.
As with every therapy, the risk of employing more aggressive COS schedules must be balanced against the benefits. However, OHSS frequencies and rates seemed to correspond between the different follitropin groups. Furthermore, the protocol modification seems effective as similar FSH doses per retrieved oocyte were observed in the age and BMI subgroups. It has also been shown that the increase in the number of retrieved eggs (i) does not decrease their quality, (ii) results in a greater number of embryos available for transfer and/or cryopreservation, (iii) increases the rates of gestation and live births, (iv) reduces the number of fresh stimulated cycles, and consequently the costs and risks involved and increases the probabilities of (v) having a baby at home and of (vi) completing the family per oocyte aspiration.8
An intern cohort of follitropin alpha-stimulated patients, with a starting dose of 300 IU, meeting the same inclusion criteria as those from the follitropin delta group, was also described as reference. The results for this group appeared to be similar to those for the Follitropin delta group.
Weaknesses and limitations
Our study has some limitations. Its retrospective nature and lack of a control group limit the ability to establish causal relationships between the modified Rekovelle® protocol and the observed outcomes. Given its descriptive nature, we have focused on retrieved oocytes’ number and maturity stage instead of clinical ICSI outcomes. Such a narrow scope of analysis limits the ability to assess the overall effectiveness of the modified protocol. Using historical cohort groups is another drawback that introduces potential bias and confounding factors that could have influenced the results. This single-center study potentially limits the findings’ generalizability to other populations or settings.
The strength of this study is that it is the first study addressing real-world practice variations in the administration of Rekovelle®, showing a satisfactory number of retrieved oocytes and maturity rates across patients in different ranges of age and BMI, without an increase in the risk of OHSS.
In conclusion, the descriptive data presented here provide a rationale for conducting clinical trials to access and confirm the benefits of using the modified Rekovelle® protocol, to improve ovarian response and cumulative clinical outcomes of ART.
Competing interests
No competing interests were disclosed.
Funding
This research received no specific grant from any public, commercial, or not-for-profit funding agency.
Author contributions
Conceptualization: Edson Borges Jr. (Equal), Daniela Braga (Equal), Amanda Setti (Equal). Data curation: Edson Borges Jr. (Equal), Daniela Braga (Equal), Patricia Guilherme (Equal), Assumpto Iaconelli Jr. (Equal), Amanda Setti (Equal). Formal Analysis: Edson Borges Jr. (Equal), Daniela Braga (Equal), Patricia Guilherme (Equal), Assumpto Iaconelli Jr. (Equal), Amanda Setti (Equal). Writing – original draft: Edson Borges Jr. (Equal), Daniela Braga (Equal), Amanda Setti (Equal). Writing – review & editing: Edson Borges Jr. (Equal), Daniela Braga (Equal), Assumpto Iaconelli Jr. (Equal), Amanda Setti (Equal). Investigation: Assumpto Iaconelli Jr. (Equal), Amanda Setti (Equal).

_in_the_modified_protocol_and_how_much_.png)



_in_the_modified_protocol_and_how_much_.png)