Introduction
The reproductive aging process in a woman occurs with the depletion of the number of oocytes or ovarian reserve.1 Decreased ovarian reserve refers to the reduced number and quality of the remaining oocytes in the ovary.2
The incidence of low ovarian reserve ranges from 6 to 64% in infertile women of different ages. In these patients, not only a reduction in the number and quality of remaining oocytes is observed, but also a decrease in the ovarian response to the administration of gonadotropins in controlled ovarian stimulation, high rate of cycle cancellation, increase in the dose of ovulation stimulants, decrease in the number of oocytes retrieved, decrease in the rate of clinical pregnancy and live birth, and high rate of spontaneous abortion in reproductive treatments highly complex assisted.2
Although a decrease in reproductive potential is considered part of the natural ovarian aging process, it can also be found in young women.2
Subsequently, various studies were carried out where it was defined that the previous diagnostic criteria were based on a heterogeneous population with different reproductive results, age was not taken into account in terms of oocyte quality and to use them it was necessary to have completed 1 stimulation cycle, reason whereby in 2019 a group of experts created a more precise classification that takes into account patient-oriented strategies according to the number of oocytes obtained individually called POSEIDON for its acronym in English. This panel of experts suggests a novel, more detailed stratification for patients with BRO, adding to the already mentioned factors, ovarian reserve in relation to the number of oocytes and aneuploidy rate in relation to maternal age, sensitivity to external gonadotrophins.3
With this objective, POSEIDON classifies patients with poor response into four groups:
-
Group 1: patients under 35 years of age with normal ovarian reserve parameters (AMH ≥1.2 ng/mL; AFC ≥5) and unexpectedly poor or suboptimal outcome to ovulation stimulation (≤9 oocytes in the 1st cycle of stimulation)
-
Group 2: patients older than 34 years with normal ovarian reserve parameters (AMH ≥1.2 ng/mL; AFC ≥5) and unexpectedly poor or suboptimal outcome to ovulation stimulation (≤9 oocytes in the 1st cycle of stimulation)
-
Group 3: patients under 35 years of age with low ovarian reserve parameters (AFC <5, AMH <1.2 ng/mL).
-
Group 4: patients older than 35 years with low ovarian reserve parameters (AFC <5, AMH <1.2 ng/mL).
With these criteria, it was possible to individualize the treatment used based on the individual characteristics of each patient, and to propose focused therapeutic strategies.
In patients classified in the POSEIDON III and IV group, multiple adjuvant treatments have been investigated to increase the number of antral follicles at the beginning of the cycle and thus increase the number of oocytes obtained in follicular capture, some of these are the administration of growth hormone, dehydroepiandrosterone and testosterone of which indisputably effective recommendations are not yet available.3 While the effects of androgens (dehydroepiandrosterone and testosterone) on follicle maturation in humans remain controversial, due to lack of direct investigations, experiments in rodents strongly demonstrate an essential function of androgens mediated by the androgen receptor (AR) on granulosa cells, especially in early stages of follicle maturation up to preantral stages.4
The hormone Dehydroepiandrosterone (DHEA) is the most potent androgen and the one with the highest affinity to bind to the androgen receptor, in women it is converted to testosterone and to a lesser extent to estradiol. DHEA supplementation for a minimum of 6 weeks is sufficient to be able to observe the beneficial effects that prove the positive effect during the early stages of follicular maturation, also coinciding with the presence of androgen receptors.4 After the administration of DHEA, Testosterone (T) levels rise, this elevation being statistically associated with the chances of pregnancy after IVF. Increased androgen levels have previously been associated with elevated anti-Müllerian hormone (AMH) levels, which are favorable for improving functional ovarian reserve.5
Testosterone, the main circulating androgen in women, is a naturally occurring steroid secreted by the ovaries and adrenal glands (100 to 400 mcg/day).5 Peak serum testosterone concentrations are reached 24-36 hours after administration, with wide interindividual variability. Testosterone is primarily metabolized in the liver by CYP3A4.
Circulating testosterone is strongly bound to plasma proteins, with about 66% bound to sex hormone-binding globulins and 33% to albumin. The free fraction of testosterone is determined by the rate of testosterone production, the rate of metabolic clearance, and the level of sex hormone-binding globulins.5
So far, the review of both clinical and preclinical evidence that the use of androgens can have a positive effect on follicular maturation is too limited to standardize their use; however, multiple published studies have shown that within of the therapeutic ranges can be clinically beneficial, mainly in those patients with low ovarian reserve and in assisted reproduction treatments.3–5
The low ovarian response is one of the daily challenges of the clinician in the infertility consultation. It is estimated that the incidence of low responders in the population undergoing IVF is 9-24% 7,10. These patients have worse pregnancy rates when compared to patients with a normal response. Numerous strategies have been proposed for the treatment of low responders, but up to now no advantages of some treatments over others have been achieved. Some recent published studies support the use of androgens as a supplement for women in these conditions, since they increase the expression of the FSH receptor in granulosa cells, promote the initiation of primordial follicle growth, and increase the number of pre-antral and antral follicles.6,7
Objectives
Main objective: To evaluate the increase in the antral follicular count in patients of the POSEIDON IV group after the administration of 50mg daily for 1 month of transdermal testosterone prior to ovarian stimulation.
Secondary objective: To evaluate the number of metaphase II oocytes after treatment with 50mg of transdermal testosterone daily for one month compared to patients who did not receive any treatment prior to ovarian stimulation.
Statistical Analyses
A quantitative, descriptive, observational, longitudinal, retrolective study was carried out in which a historical cohort was analyzed in the assisted reproduction clinical research area at the Hisparep Clinic of Hospital Español de México.
An analysis with descriptive statistics was performed for the baseline characteristics of both groups. Then a chi-square test was performed to obtain statistically significant variables. A p value <0.05 was used to be considered significant, for the descriptive analysis, frequencies and proportions were used for the categorical variables and measures of central tendency and dispersion for the numerical variables. According to normality and homogeneity, parametric or non-parametric tests were used to compare before and after.
RESULTS
The women who will be included in the protocol must be over 35 years of age who have attended Hisparep Assisted Reproduction Clinic for assisted reproduction treatment, either with primary or secondary infertility and meet the classification criteria of the POSEIDON IV group. (age > or = 35 years with CFA < 5 follicles or AMH < 1.2 ng/mL) and in the first group of patients they have been given supplementation with transdermal Testosterone 50 mg one month prior to ovarian stimulation. In the second group, they have not received treatment before ovarian stimulation.
Material and methods:
The treatment protocol consisted of providing a fixed dose of transdermal testosterone which was 50 mg (1 gel) applied to the forearm every 24 hours, 30 days before starting the next menstrual cycle in conjunction with ovarian stimulation.
This study has the approval of the ethics committee of the HISPAREP clinic. Each patient was given an informed consent which they signed before the study. We declare that we have no conflict of interest.
Twenty patients belonging to the POSEIDON IV group were selected, of which 10 received supplementary treatment with testosterone 50 mg transdermal daily for one month prior to ovarian stimulation and 10 did not receive testosterone; whose general characteristics are mean age of 40.2 ± 2.5 years of the group that received testosterone and 43.3 ± 2 years for the group that did not receive testosterone, while normal weight was found in 80% and 90% (overweight of 20% and 10% ) of each group, respectively (Table 1).
The initial conditions of the patients are described below: AMH (mean ± standard deviation) of 0.65 ± 0.28 ng/dL in those who received testosterone and 0.84 ± 0.49 ng/dL in those who did not receive testosterone (Figure 1); the altered infertility factor was ovarian endocrine in 60% (n=6) of the participants who were administered testosterone and in 40% (n=4) of those who were not administered, in both this cause represented mode (Figure 2).
The most common type of stimulation received was with recombinant FSH/LH in both groups with GnRH antagonist.
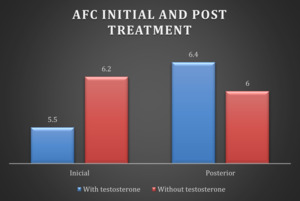
The antral follicular count observed by ultrasound after testosterone treatment was 6.4 ± 2.4, without testosterone 6 ± 3.47; p<0.778. Without observing significant differences (Figure 3).
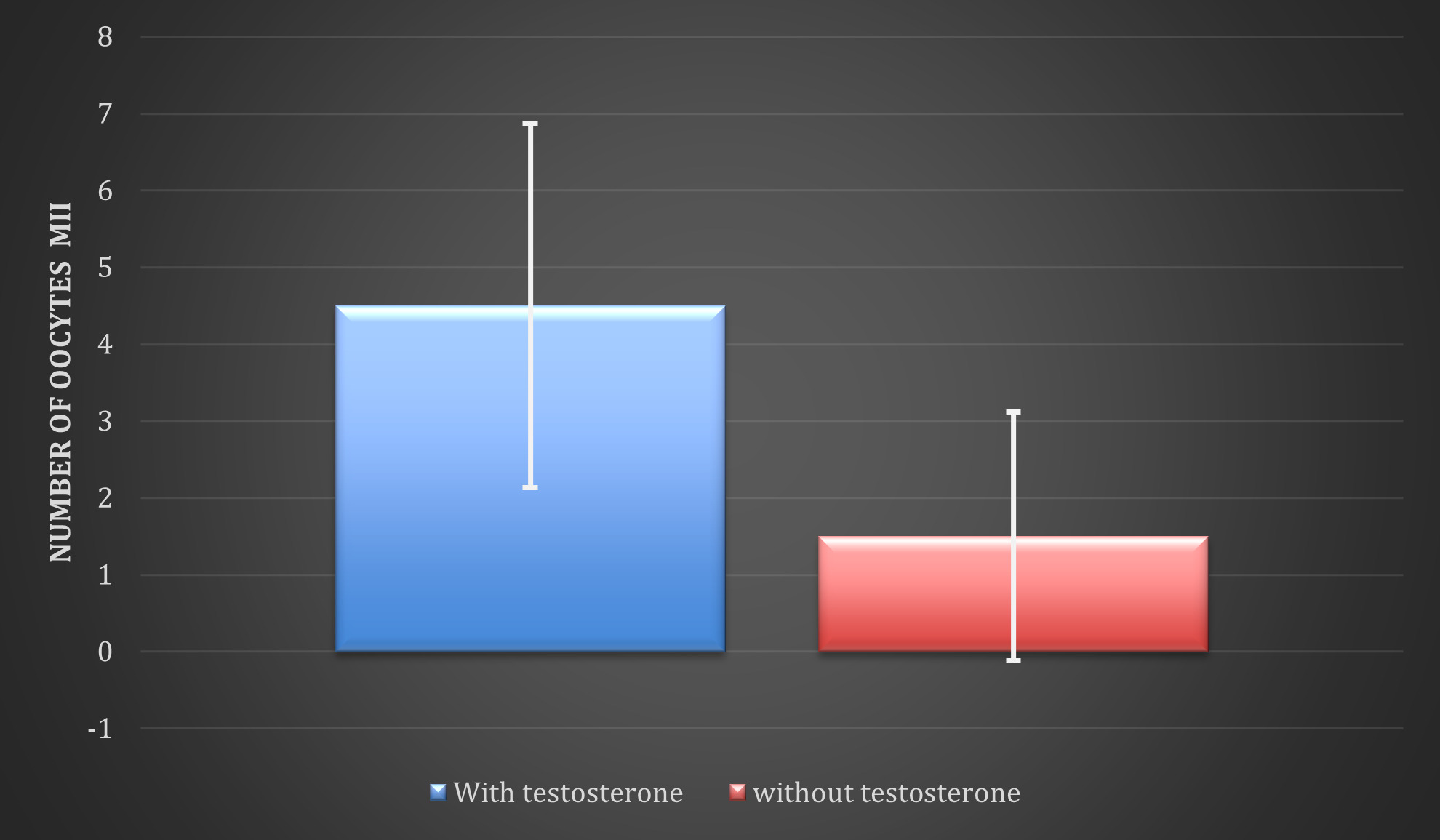
The number of metaphase II oocytes obtained (mean ± standard deviation) after testosterone administration was 4.5 ± 2.37 and 1.5 ± 1.62 in the participants who did not receive testosterone (Figure 4); When carrying out a statistical analysis in which the count of oocytes in metaphase II observed after the administration of testosterone and the count in the absence of testosterone was compared through the Chi square, with 7 degrees of freedom, it was obtains that p=0.04886, that is p<0.05, so the results were statistically significant in favor of the administration of testosterone.
Discussion
The decrease in ovarian reserve has become one of the leading causes of infertility, found in 9 to 24% of patients undergoing assisted reproduction treatment,8 this is mainly due to the current tendency to delay pregnancy at older ages. A close relationship has been found between patients with decreased ovarian reserve and low response to stimulation treatment.9 In our clinic, the patients with decreased ovarian reserve were around 60% of all patients because most of them were more than 40 years old.
Gleicher mentions in his study that women over the age of 35 may benefit from a more direct administration of testosterone (transdermal testosterone) due to the less efficient conversion rate of DHEA to testosterone.4 A meta-analysis by Bosdou et al. reports that the clinical pregnancy rate was significantly increased by 15% in patients who were pretreated with transdermal testosterone compared with those who were not (+15%, 95% CI).10 Similarly, the live birth rate increased by 11% in patients pre-treated with transdermal testosterone (11%, 95% CI).10
According to the theory underlying the literature, intraovarian androgens promote FSH sensitivity in growing follicles and, therefore, could increase performance and oocyte maturity after ovarian stimulation and improve pregnancy rates.11 In this context, it should be noted that in all the interventions analyzed in the Bosdou meta-analysis, the effect in terms of clinical outcomes of pregnancy rates is only observed as statistically significant in the case of transdermal testosterone compared to other therapies, such as the addition aromatase inhibitors or hCG.10
Two small studies6,7 built on the proposed theory to enhance follicular development with increased intraovarian androgens.11 The hypotheses that are postulated and evaluated for effective treatment in patients with BRO are:
-
Increase the number of oocytes retrieved
-
decrease of the total dose of gonadotropins required
-
Decrease the duration of ovarian stimulation.
Regarding the antral follicular count, an increase was also observed. However, this result was not statistically significant, so it is still necessary to carry out randomized clinical trials with a larger sample size to increase the evidence of this type of treatment and use it in a more effective way routine in this group of patients, which continues to be a challenge for the doctor in the area of assisted reproduction.
Only in the case of transdermal testosterone administrations were all these hypotheses confirmed. Currently, based on the limited evidence available, transdermal testosterone pretreatment appears to increase clinical pregnancy and survival relative to birth rates in poor responders undergoing ovarian stimulation for in vitro fertilization.9,12
Some of the limitations of this study were the relatively small sample size (20 participants), it was conducted at a single breeding center, the long-term effects of testosterone supplementation on reproductive results and possible risks or side effects and results in clinical pregnancy rate and live birth rate were not evaluated.
Conclusions
The poor response to an ovarian stimulation cycle in women in the POSEIDON IV group could be improved with supplementary treatments such as transdermal testosterone. The dose of 50 mg every 24 hours for a month before the ovarian stimulation increases the number of metaphase II oocytes recovered statistically significantly.
For this reason, we propose that the reproductive results in women with poor ovarian reserve within group IV of POSEIDON will be favorably modified after the administration of 50mg of testosterone administered dermal daily for one month before ovarian stimulation. However, it is still necessary to carry out randomized clinical trials that can support its routine use in this group of patients who continue to be a challenge for doctors in assisted reproduction treatment.
Authors’ Contribution
Conceptualization: Martha Esmeralda Espinosa Esparza; Data curation: Jorge Luis Lezama Ruvalcaba; Formal Analysis: José Carlos Salazar Trujillo; Funding acquisition: Martha Esmeralda Espinosa Esparza; Investigation: Martha Esmeralda Espinosa Esparza; Methodology: Martha Esmeralda Espinosa Esparza; Project administration: Carlos Gerardo Salazar López Ortíz; Resources: Martha Esmeralda Espinosa Esparza; Software: Jorge Luis Lezama Ruvalcaba; Supervision: Carlos Gerardo Salazar López Ortíz; Validation: Jorge Luis Lezama Ruvalcaba; Visualization: Martha Esmeralda Espinosa Esparza; Writing – original draft: Martha Esmeralda Espinosa Esparza; Writing – review & editing: Jorge Luis Lezama Ruvalcaba.

.png)
.png)

.png)
.png)